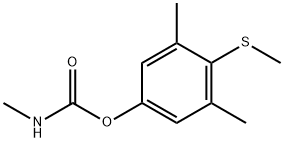
Mercaptodimethur
- Product NameMercaptodimethur
- CAS2032-65-7
- CBNumberCB1254073
- MFC11H15NO2S
- MW225.31
- EINECS217-991-2
- MDL NumberMFCD00055450
- MOL File2032-65-7.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 119°C |
| Density | 1.1838 (rough estimate) |
| vapor pressure | 1.5 x 10-5 Pa (20 °C) |
| refractive index | 1.4790 (estimate) |
| storage temp. | 0-6°C |
| solubility | DMF: 20 mg/ml,DMSO: 20 mg/ml,DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml,Ethanol: 10 mg/ml |
| Boiling point | 310.7±42.0 °C(Predicted) |
| pka | 12.16±0.46(Predicted) |
| form | Crystalline Solid |
| color | White |
| Water Solubility | Insoluble |
| BRN | 1881431 |
| Stability | Light Sensitive |
| CAS DataBase Reference | 2032-65-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | JI9431OS31 |
| NIST Chemistry Reference | 4-Methylthio-3,5-xylyl methylcarbamate(2032-65-7) |
| EPA Substance Registry System | Methiocarb (2032-65-7) |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H300-H410 | |||||||||
| Precautionary statements | P264-P270-P273-P301+P310-P391-P405 | |||||||||
| Hazard Codes | T;N,N,T | |||||||||
| Risk Statements | 25-50/53 | |||||||||
| Safety Statements | 22-37-45-60-61 | |||||||||
| RIDADR | UN 2811/2757 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FC5775000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 2032-65-7(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in male, female rats (mg/kg): 70, 60 orally (Gaines) | |||||||||
| NFPA 704: |
|


