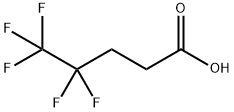
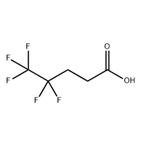
Synthesis
4,4,5,5,5-Pentaflfluoropentanol (1.8 kg, 10.1 mol), tetraethylammonium hydrogen sulfate (18.1 g, 0.08 mole), and water (10.8 L) were added to a 50 L QVF vessel and heated with stirring to 70 °C. Sodium permanganate monohydrate (2.33 kg, 14.14 mol) was dissolved at 20 °C in water (10.8 L) and transferred to a measure vessel. Aqueous sodium permanganate was added in aliquots (approximately 10% at a time) to the stirred aqueous solution of pentaflfluoropentanol and tetraethylammonium hydrogen sulfate maintaining a temperature of 65−75 °C by the additions of permanganate. The total time taken to add the aqueous permanganate was 2 h 30 min. The reaction was stirred at 70 °C for 4 h when gas chromatography (GC) analysis showed conversion of pentaflfluoropentanol to pentaflfluoropentanoic acid to be complete. The reaction mixture was allowed to cool to ambient temperature overnight and screened through a Celite fifilter aid (500 g) to remove precipitated manganese dioxide. The isolated manganese dioxide was washed with hot water (60 °C, 18 L). The combined aqueous layers were extracted with methyl tert-butyl ether (5.4 L), and the upper organic layer was discarded. The aqueous layer was acidifified with concentrated sulfuric acid (320 mL) to pH 1. The lower organic layer which separated was retained. The aqueous layer was extracted with methyl tert-butyl ether (2 × 5.4 L), and the upper organic layers were combined with the initial, lower organic layer. The combined organic layers were washed with water (5.4 L) and dried with anhydrous sodium sulfate. The organic solvent was removed in vacuo at 50 °C and the residue distilled to give the acid as a pale pink, low-melting solid (1.49 kg, 77%).
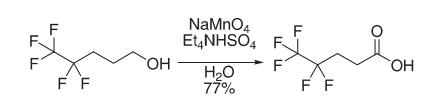
Reference: Mahmood, A.; Robinson, G. E.; Powell, L. Org. Proc. Res. Dev. 1999, 3, 363–364.