Manufacturing Process
Hydrogenation of 4-diisopropylamino-2-phenyl-2-pyridin-2-yl-butyramide over
platinum oxide catalyst reduced the pyridine ring to a piperidine to give 4-
diisopropylamino-2-phenyl-2-piperidin-2-yl-butyramide as a white solid, MP:
107°-108°C. Structure was confirmed by proton, carbon-13-NMR spectra and
by elemental analysis.
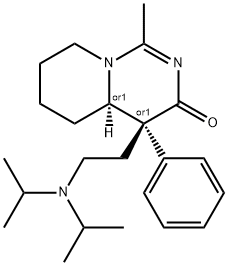
2-(1-Acetylpiperidin-2-yl)-4-diisopropylamino-2-phenylbutyramide was
prepared by acetylation of above product with N,N-dimethylacetamide
dimethylacetal by heating at 80°C for about 14 hours. The acetamide melted
at 191°-192°C. The treatment of that intermediate with lithium aluminum
hydride led the newly introduced acetyl group to condense with the adjacent
amide nitrogen. There was thus obtained 4-(2-diisopropylaminoethyl)-1-
methyl-4-phenyl-4,4a,5,6,7,8-hexahydropyrido[1,2-c]pyrimidin-3-one, the
new anti-arrhythmic agent actisomide, MP: 70°-75°C. Its structure was
confirmed by proton NMR and infrared spectra and by elemental analysis.