Background
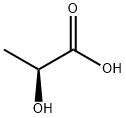
L-Lactic acid (L(+)-Lactic Acid) is the optically active form of lactic acid and has an (S) stereochemical arrangement. L-Lactic Acid was first registered as a pesticide 1988 as a plant-growth regulator. There are currently no L-Lactic Acid end-use pesticide products for use as a plant growth regulator. Animals produce L-Lactate, the conjugate base of lactic acid, from pyruvate via lactate dehydrogenase in fermentation during normal metabolism and exercise. Lactic acid bacteria perform lactic acid fermentation, converting carbohydrates to lactic acid. In humans, lactic acid and its conjugate base are part of many biological processes, from prenatal development to extracellular fluid surrounding neurons. L(+)-lactic acid is a colourless to yellowish, nearly odourless, syrupy liquid with a mild acid taste. It is commercially available as aqueous solutions of various concentrations. These solutions are stable under normal storage conditions.Lactic acid is nontoxic to humans and the environment, but concentrated solutions can cause skin irritation and eye damage. Thus, they must be labelled with a hazard pictogram and related statements. Lactic acid is readily biodegradable.
Chemical Properties
Crystalline Solid
Uses
Lactic acid has been used:
- as a component in substrate solution II for lactate dehydrogenase reaction
- as an additive in storage solution A
- as a supplement in the artificial gastric juice preparation for evaluation of degree of resistance Lactobacillus to the gastric stresses
Uses
It is most commonly used for fluid resuscitation after blood loss due to trauma, surgery, or burn injury. It is used in the agricultural, chemical, leather processing, pharmaceuticals, and cosmetics industries, used as an electroplating agent, food/feed additive, pH regulator, cleaning/washing agent, and tanning agent; used to flavor animal feeds; used as a solvent and intermediate in chemical production, as a pH regulator in fabric finishing, and in paints, coatings, soaps, and cleaning products; used to make large scale and fine chemicals, pulp-paper-paper products, food products, and plastic products, in mining, health services, agriculture-forestry-fishing, and building and construction work.
Uses
Occurs in small quantities in the blood and muscle fluid of man and animals. The lactic acid concentration increases in muscle and blood after vigorous activity. L-(+)-Lactic acid is also present in liver, kidney, thymus gland, human amniotic fluid, and o
Definition
ChEBI: An optically active form of lactic acid having (S)-configuration.
General Description
L-(+)-Lactic acid is the only naturally occurring lactic acid in humans and mammals. Commercially, few bacteria like
Lactobacillus casei,
L. delbrueckii,
Streptococcus lactis produces L-Lactic acid by fermentation process. Lactic acid activates hydroxycarboxylic acid receptor, G-protein coupled receptor 81 (GPR81).
Flammability and Explosibility
Not classified
Biochem/physiol Actions
L-(+)-Lactic acid is used as a substrate for lactic acid dehydrogenase and lactate oxidase.
Purification Methods
Purify lactic acid by fractional distillation at 0.1mm pressure, followed by fractional crystallisation from diethyl ether/isopropyl ether (1:1, dried with sodium). [Borsook et al. J Biol Chem 102 449 1933.] The solvent mixture, *benzene/diethyl ether (1:1) containing 5% pet ether (b 60-80o) has also been used. [Brin Biochemical Preparations 3 61 1953, Beilstein 3 IV 633.]