Synthesis
Step C: Synthesis of 4-(1H-pyrazol-1-yl)piperidine (i-4)
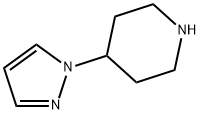
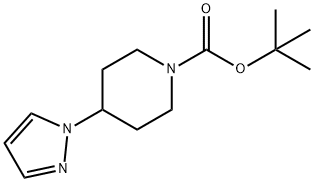
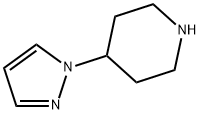
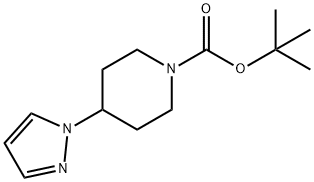
The tert-butyl 4-(1H-pyrazol-1-yl)piperidine-1-carboxylate (50 mg, 0.2 mmol) obtained from Step B was dissolved in a dioxane solution (2.0 mL) of 4M HCl. The reaction mixture was stirred at room temperature for 1 hour. Subsequently, the solvent was removed by concentration under reduced pressure and dried under high vacuum to afford the white solid product 4-(1H-pyrazol-1-yl)piperidine (i-17) (30 mg, 96% yield). The product was analyzed by ESI-MS and the calculated value (C8H13N3) was 151.11 and the measured value was 151.11 [M+H]+, found to be 152.10.
References
[1] Patent: EP2632457, 2017, B1. Location in patent: Paragraph 0088; 0094
[2] Patent: WO2013/10453, 2013, A1. Location in patent: Page/Page column 95