Synthesis
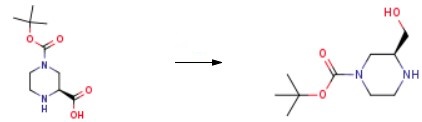
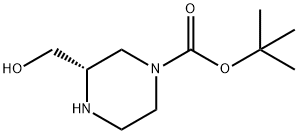
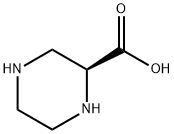
To synthesize (S)-1-Boc-3-hydroxymethyl-piperazine, a stirred suspension of (S)-piperazine-l,3-dicarboxylic acid l-tert-buty ester (5.00 g, 21.7 mmol) in THF (40 mL) was prepared. Slowly, 1.0 M borane-THF complex solution (32.6 mL, 32.6 mmol) was added to the suspension. The reaction mixture was heated to 90 ℃ and stirred under reflux for 2 h. Before further proceeding, the mixture was removed from heat and an additional 1.5 equivalents of 1.0 M borane-THF complex solution (32.6 mL, 32.6 mmol) was added. The reaction was then reheated and stirred under reflux for 2 h. Subsequently, the reaction mixture was cooled to 0 ℃ and quenched by slowly adding methanol. The resulting mixture was concentrated under reduced pressure, yielding a white solid. This solid was dissolved in THF (30 mL) and cooled to 0 ℃. Slowly, a 2.0 M solution of lithium aluminum hydride (LiAlH4) in THF (27 mL, 54.0 mmol) was added. The reaction was heated to 90 ℃ and stirred under reflux for 2 h. Another portion of 2.0 M LiAlH4 was added, and the reaction mixture was stirred under reflux for 4 hours, followed by overnight stirring at RT. The reaction mixture was then cooled to 0 ℃ and quenched by slowly adding 1.0 M aqueous sodium hydroxide (NaOH) solution until the exothermic reaction subsided. The product (S)-1-Boc-3-hydroxymethyl-piperazine was obtained after purification.