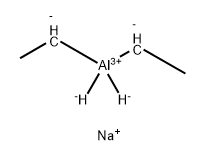
Chemical Properties
Sodium diethyldihydridoaluminate, NaAlH2(C2H5)2, is a white, crystalline substance, mp 85 – 87℃, which ignites spontaneously in air. It is insoluble in aliphatic but fairly soluble in aromatic hydrocarbon solvents. The solubility of sodium diethyldihydridoaluminate in toluene can be considerably increased by addition of a small amount of THF. Solutions of this reagent must be kept under an inert atmosphere, since they lose their activity by hydrolysis and oxidation if exposed to air. The reducing power of this hydride is comparable with that of LiAlH4. The ethyl groups generally do not take part in the reduction, but give rise to large volumes of ethane when the intermediate aluminate complex is hydrolyzed. This ethane should be safely disposed of, preferably by burning.
Preparation
Sodium diethyldihydridoaluminate is produced by reaction of sodium, aluminum, and triethyl aluminum in toluene at ca.
150℃and 7 MPa hydrogen pressure.
3 Na+Al+2 Al(C2H5T3)3 H2→3 NaAlH2(C2H5)3