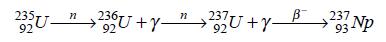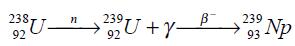Description
ML300 is a selective non-covalent inhibitor of SARS-CoV 3CL protease (IC50 = 4.11 μM).1,2
Chemical Properties
silvery metal; α: ortho-rhomb, a=0.4721 nm, b=0.4888 nm, c=0.6661nm, stable from room temp to 280°C; β: tetra, a=0.4895 nm, c=0.3386nm, stable from 280–577°C; γ: a=0.3518 nm; bcc, stable from 577–637°C; enthalpy of vaporization 418 kJ/mol; enthalpy of fusion 3.20 kJ/mol; 237Np, t1/2=2.14×10+6 years, t1/2 of 236Np 1.29×10+6 years; discovered in 1940; produced in kg amounts as a by-product of plutonium production [MER06] [KIR78] [CRC10]
Physical properties
The chemistry of neptunium (
93Np) is somewhat similar to that of uranium (
92U) and plutonium(
94Pu), which immediately precede and follow it in the actinide series on the periodictable. The discovery of neptunium provided a solution to a puzzle as to the missing decayproducts of the thorium decay series, in which all the elements have mass numbers evenlydivisible by four; the elements in the uranium series have mass numbers divisible by fourwith a remainder of two. The actinium series elements have mass numbers divisible by fourwith a remainder of three. It was not until the neptunium series was discovered that a decayseries with a mass number divisible by four and a remainder of one was found. The neptuniumdecay series proceeds as follows, starting with the isotope plutonium-241: Pu-241→Am-241→Np-237→Pa-233→U-233→Th-229→Ra-225→Ac-225→Fr-221→At-217→Bi-213→Ti-209→Pb-209→Bi-209.
Neptunium is a silvery-white radioactive, heavy metal. Its melting point is 644°C, its boilingpoint is 3,902°C, and its density is 20.25g/cm
3.
Isotopes
There are a total of 23 isotopes of neptunium. None are stable. All are radioactivewith half-lives ranging from two microseconds to 2.144×10
+6years for the isotopeNp-237, which spontaneously fissions through alpha decay.
Origin of Name
Named for the planet Neptune.
Occurrence
At one time, neptunium’s entire existence was synthesized by man. Sometime later, in themid-twentieth century, it was discovered that a very small amount is naturally produced inuranium ore through the actions of neutrons produced by the decay of uranium in the orepitchblende. Even so, a great deal more neptunium is artificially produced every year than everdid or does exist in nature. Neptunium is recovered as a by-product of the commercial productionof plutonium in nuclear reactors. It can also be synthesized by bombarding uranium-238with neutrons, resulting in the production of neptunium-239, an isotope of neptunium witha half-life of 2.3565 days.
History
Neptunium
was the first synthetic transuranium element of the actinide
series discovered; the isotope
239Np was produced by McMillan
and Abelson in 1940 at Berkeley, California, as the result of
bombarding uranium with cyclotron-produced neutrons. The
isotope
237Np (half-life of 2.14 × 106 years) is currently obtained
in gram quantities as a by-product from nuclear reactors in
the production of plutonium. Twenty-three isotopes and isomers
of neptunium are now recognized. Trace quantities of
the element are actually found in nature due to transmutation
reactions in uranium ores produced by the neutrons which are
present. Neptunium is prepared by the reduction of NpF3 with
barium or lithium vapor at about 1200°C. Neptunium metal
has a silvery appearance, is chemically reactive, and exists in
at least three structural modifications: α-neptunium, orthorhombic,
density 20.25 g/cm3, β-neptunium (above 280°C),
tetragonal, density (313°C) 19.36 g/cm3; γ-neptunium (above
577°C), cubic, density (600°C) 18.0 g/cm3. Neptunium has four
ionic oxidation states in solution: Np+3 (pale purple), analogous
to the rare earth ion Pm+3, Np+4 (yellow green); NpO+ (green
blue); and NpO++ (pale pink). These latter oxygenated species
are in contrast to the rare earths that exhibit only simple ions
of the (II), (III), and (IV) oxidation states in aqueous solution.
The element forms triand tetrahalides such as NpF3, NpF4,
NpCl4, NpBr3, NpI3, and oxides of various compositions such
as are found in the uranium-oxygen system, including Np
3O
88
and NpO2.
Characteristics
Neptunium is the first of the subseries of the actinide series known as the transuranic elements—those heavy, synthetic (man-made) radioactive elements that have an atomic numbergreater than uranium in the actinide series of the periodic table. An interesting fact is thatneptunium was artificially synthesized before small traces of it were discovered in nature. Moreis produced by scientists every year than exists in nature.
Neptunium has an affinity for combining with nonmetals (as do all transuranic elements)such as oxygen, the halogens, sulfur, and carbon.
Uses
The most important radioactive isotope of neptunium is Neptunium-237, with a half-lifeof 2.144×10
+6years, or about 2.1 million years, and decays into protactinium-233 throughalpha decay. Neptunium’s most important use is in nuclear research and for instrumentsdesigned to detect neutrons.
Uses
Source material for production of 238U (power source).
Definition
neptunium: Symbol Np. A radioactivemetallic transuranic elementbelonging to the actinoids; a.n. 93;r.a.m. 237.0482. The most stable isotope,neptunium–237, has a half-lifeof 2.2×10
6 years and is produced insmall quantities as a by-product bynuclear reactors. Other isotopes havemass numbers 229–236 and 238–241.The only other relatively long-livedisotope is neptunium–236 (half-life 5×10
3 years). The element was firstproduced by Edwin McMillan (1907–91) and Philip Abelson (1913–2004) in1940.
Definition
A toxic radioactive silvery element of the actinoid series of metals that was the first transuranic element to be synthesized (1940). Found on Earth only in minute quantities in uranium ores, it is obtained as a by-product from uranium fuel elements. Symbol: Np; m.p. 640°C; b.p. 3902°C; r.d. 20.25 (20°C); p.n. 93; most stable isotope
237Np (half-life 2.14 × 10
6 years).
Definition
A radioactive transuranic element having atomic number 93, first formed by
bombarding uranium with high-speed deuterons
aw 237.0482, valences of 3, 4, 5, 6; d 20.45.
Neptunium-237, the longest-lived of the 11 isotopes, has been found naturally in extremely
small amounts in uranium ores. It is produced in
weighable amounts as a by-product in the production of plutonium.
Production Methods
Neptunium-237 is obtained as a by-product of making plutonium from uranium isotopes in nuclear reactors. Significant amounts of this element may be recovered from plutonium plant nuclear wastes. Both the recovery and purification of neptunium can be carried out by various chemical processes, including precipitation, solvent extraction and ion exchange.
Neptunium-237 may be synthesized by bombarding uranium-235 or uranium-238 with neutrons:
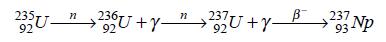
Neptunium-239 may be obtained from uranium-238 by neutron bombardment as it was first produced:
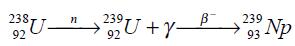
Neptunium may be prepared in the metallic state by the reduction of its trifluoride with barium vapor at 1,200°C followed by rapid cooling. Its tetrafluoride may be reduced with excess calcium metal at about 750°C under argon atmosphere.
Hazard
A radioactive poison.
Hazard
All isotopes of neptunium are highly radioactive and are hazardous and thus need to becarefully used in controlled laboratory settings. These isotopes as well as neptunium’s compoundsare radioactive poisons.
References
Turlington et al. (2012) Non-covalent triazole-based inhibitors of the SARS main protease 3CLpro; In: Probe Reports from the NIH Molecular Libraries Program
Turlington et al. (2013) Discovery of N-(Benzo[1,2,3]triazol-1-yl)-N-(benzyl)acetamido)phenyl)carboxamides as severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro inhibitors: Identification of ML300 and noncovalent nanomolar inhibitors with an induced-fit binding; Bioorg. Med. Chem. Lett. 23 6172