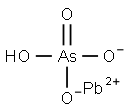
LEAD ARSENATE
- Product NameLEAD ARSENATE
- CAS7784-40-9
- CBNumberCB0763062
- MFAsHO4Pb
- MW347.13
- EINECS232-064-2
- MDL NumberMFCD00054015
- MOL File7784-40-9.mol
Chemical Properties
| Melting point | decomposes at 1042℃ [HAW93] |
| Density | 5.79 |
| solubility | insoluble in H2O; soluble in HNO, alkaline solutions 3 |
| form | white monoclinic crystals |
| color | white monoclinic crystals, crystalline |
| Water Solubility | insoluble H2O; soluble HNO3, alk [CRC10] |
| Solubility Product Constant (Ksp) | pKsp: 35.39 |
| CAS DataBase Reference | 7784-40-9 |
| FDA UNII | A9AI2R9EWN |
| EPA Substance Registry System | Lead(II) arsenate (1:1) (7784-40-9) |
Safety
| Symbol(GHS) |
  
|
| Signal word | Danger |
| Hazard statements | H301-H331-H350-H360Df-H373-H410 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P261-P271-P304+P340-P311-P321-P403+P233-P405-P501-P260-P314-P501-P273-P391-P501 |
| Hazard Codes | T,N |
| Risk Statements | 45-61-23/25-33-50/53-62 |
| Safety Statements | 53-45-60-61 |
| RIDADR | 1617 |
| HazardClass | 6.1 |
| PackingGroup | II |
| HS Code | 28429090 |
| Hazardous Substances Data | 7784-40-9(Hazardous Substances Data) |
| Toxicity | LD50 in rats, rabbits (mg/kg): 825, 125 orally (Voigt); LD50 in female rats (mg/kg): 1050 orally, >2400 dermally (Gaines) |