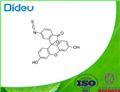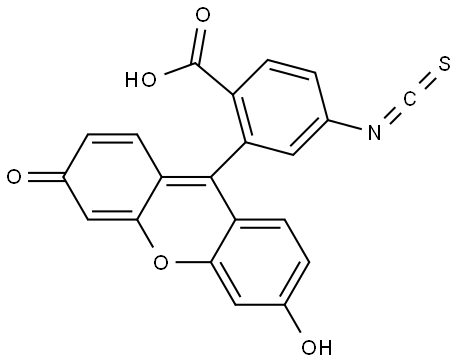
6-FITC
- Product Name6-FITC
- CAS3012-71-3
- CBNumberCB0702517
- MFC21H11NO5S
- MW389.38
- MDL NumberMFCD00041838
- MOL File3012-71-3.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 741.1±60.0 °C(predicted) |
| Density | 1.46±0.1 g/cm3(Temp: 20 °C; Press: 760 Torr)(predicted) |
| storage temp. | 2-8°C |
| form | lyophilized powder |
| pka | 4.02±0.10(predicted) |
| color | Light yellow to yellow |
| biological source | sheep |
| UNSPSC Code | 12352203 |
| NACRES | NA.46 |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H302-H311-H411 |
| Precautionary statements | P280-P301+P312+P330-P302+P352+P312 |
| Hazard Codes | Xi |
| Risk Statements | 36-42/43 |
| Safety Statements | 26-45-36/37-22 |
| WGK Germany | 3 |
| HS Code | 29329990 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Dermal Acute Tox. 4 Oral Aquatic Chronic 2 |