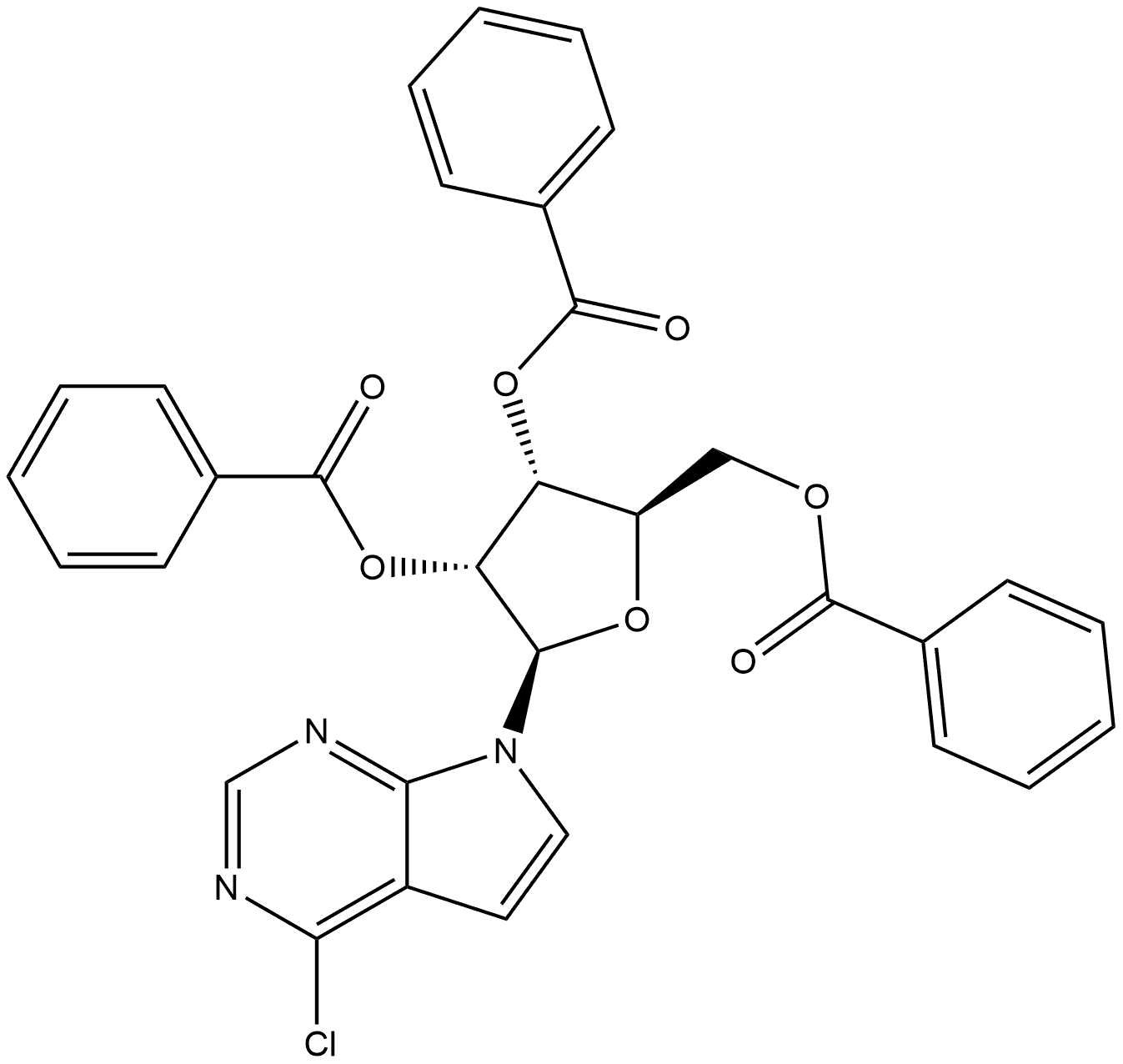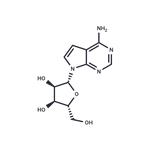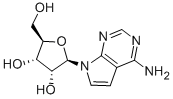
TUBERCIDIN
- Product NameTUBERCIDIN
- CAS69-33-0
- CBNumberCB0461137
- MFC11H14N4O4
- MW266.25
- EINECS200-703-4
- MDL NumberMFCD00056012
- MOL File69-33-0.mol
Chemical Properties
| Melting point | 247-248 °C (decomp)(Solv: water (7732-18-5)) |
| alpha | D17 -67° (50% acetic acid) |
| Boiling point | 409.46°C (rough estimate) |
| Density | 1.2896 (rough estimate) |
| refractive index | 1.8340 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Soluble in DMSO |
| form | powder |
| pka | 12.44±0.70(Predicted) |
| color | off-white |
| Merck | 13,9875 |
| BRN | 38498 |
| Stability | Hygroscopic |
| InChIKey | HDZZVAMISRMYHH-UIRWVKCDSA-N |
| CAS DataBase Reference | 69-33-0 |
| EWG's Food Scores | 1 |
| FDA UNII | M351LCX45Y |
| NCI Drug Dictionary | tubercidin |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H300 | |||||||||
| Precautionary statements | P264-P301+P310 | |||||||||
| Hazard Codes | T+ | |||||||||
| Risk Statements | 28 | |||||||||
| Safety Statements | 36/37/39-45 | |||||||||
| RIDADR | UN 3462 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UY8870000 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29419090 | |||||||||
| Toxicity | LD50 i.v. in mice: 45 mg/kg (Anzai) | |||||||||
| NFPA 704: |
|