Synthesis
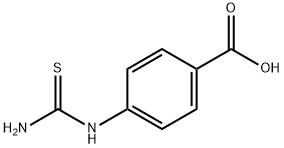
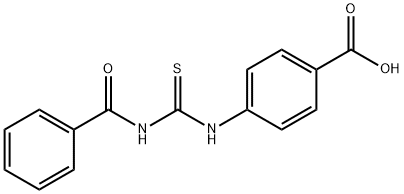
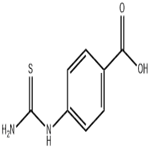
4-(3-Benzoylthioureido)benzoic acid (7) (10.0 mmol) was used as raw material and mixed with 10% sodium hydroxide solution (10.0 mmol) in 20 mL of water. The reaction mixture was heated to reflux for 30 min. After completion of the reaction, it was cooled to room temperature and neutralized with hydrochloric acid. The resulting solid product was collected by filtration, washed with water, dried and recrystallized from butanol to give (4-carboxyphenyl)thiourea in white powder form in 93% yield. The product was characterized by the following data: melting point >300 °C; infrared spectra (KBr, cm-1) νmax 3440, 3339, 3279, 3170, 1684, 1291; NMR hydrogen spectra (300 MHz, DMSO-d6) δ 12.72 (1H, br s, COOH), 9.96 (1H, s, NH), 7.54-7.90 (4H, m, ArH), 3.37 (1H, s, NH2); mass spectra (EI, 70 eV) m/z 196 (10), 179 (98), 162 (98), 134 (75), 120 (95), 92 (35); calculated elemental analysis values (C8H8N2O2S, 196.23): C, 48.97; H, 4.11; N 14.28; measured values: C, 48.84; H, 4.07; N, 14.24.
References
[1] Research on Chemical Intermediates, 2017, vol. 43, # 11, p. 6299 - 6315
[2] Journal of the American Chemical Society, 1934, vol. 56, p. 1408
[3] Journal of Medicinal Chemistry, 1997, vol. 40, # 12, p. 1901 - 1905
[4] European Journal of Medicinal Chemistry, 2015, vol. 102, p. 387 - 397
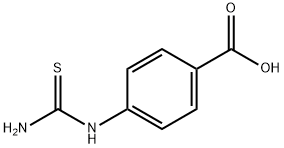

![4-[[(Benzoylamino)thioxomethyl]amino]benzoic acid](/CAS/20180906/GIF/40611-30-1.gif)