Description
(–)-Ropivacaine is a potent and reversible blocker of sodium channels in nerve fibers.
In vivo, (–)-ropivacaine induces complete impairment of proprioception, motor function, and nociception in the hindleg of rats when 100 μL of an 8 mM solution is injected percutaneously into the sciatic nerve. (–)-Ropivacaine depresses myocardial contractile force in isolated rat hearts less potently than (±)-ropivacaine, as well as (–)- and (±)-bupivacaine . Formulations containing (–)-ropivacaine have been used as local anesthetics during surgery and childbirth.
Chemical Properties
White Solid
Uses
Ropivacaine hydrochloride monohydrate has been used as an analyte in tandem mass spectrometry and as an amide-based local anaesthetic to test its effect on breast cancer cell methylation.
Definition
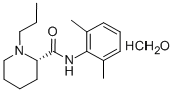
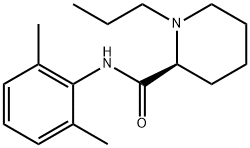
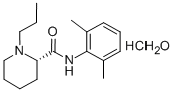
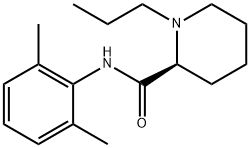
ChEBI: The monohydrate form of (S)-ropivacaine hydrochloride.
General Description
Ropivacaine has lidocaine-related structure with tertiary amine side chains.?It is a pure
S(-)enantiomer with a propyl group on the piperidine nitrogen atom.
Biochem/physiol Actions
Local anaesthetic with less cardiotoxicity than bupivacaine; causes reversible blockade of impulse propagation along nerve fibres by preventing the inward movement of sodium ions through the cell membrane of the nerve fibers.
Synthesis
The general procedure for the synthesis of (S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidine carboxamide hydrochloride hydrate using (S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide as raw material was as follows: about 3 g of (S)-N-(2,6-dimethylphenyl)-1 -propyl-2-piperidine carboxamide was dissolved in 10 mL of deionized water and to this solution, 50 mL of acetone preheated to 60°C was slowly added. Under stirring conditions, concentrated hydrochloric acid was added dropwise to adjust the pH to 2.0. Subsequently, the reaction mixture was heated to reflux until a clarified solution was formed. 20 mL of acetone was added to the reaction system and the precipitated solid product was filtered after cooling to room temperature. The solid was washed three times with cold acetone and dried in a vacuum drying oven at 40°C for 12 h. 2.9 g of white crystalline (S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride hydrate was obtained in 82% yield. The water content of the product was 5.6% as determined by Karl Fischer's method.
References
[1] Patent: US2006/276654, 2006, A1. Location in patent: Page/Page column 5
[2] Patent: US2006/276654, 2006, A1. Location in patent: Page/Page column 5