Uses
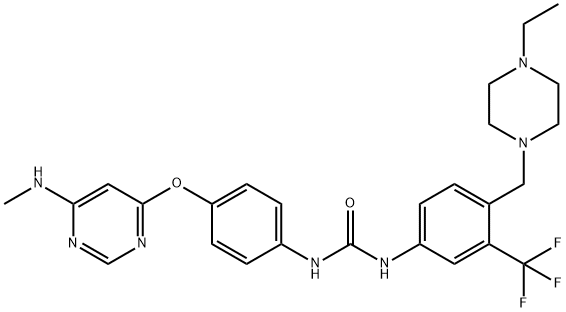
N-[4-[(4-Ethyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]-N''-[4-[[6-(methylamino)-4-pyrimidinyl]oxy]phenyl]urea is an inhibitor of RET, FLT3, KDR, c-Abl and c-Kit. Also, it prevent the growth of human thyroid cancer cells. It is a COVID19-related research product.
Uses
AST-487 is an inhibitor of RET (IC50 = 0.88 μM), FLT3 (Ki = 0.52 μM), KDR (IC50 = 0.17 μM), c-Abl (IC50 = 0.02 μM), and c-Kit (IC50 = 0.5 μM). It has been shown to inhibit RET autophosphorylation and activation of downstream effectors and to prevent the growth of human thyroid cancer cell lines with activating mutations of RET but not of lines without RET mutations. In xenografts of NIH3T3 cells expressing oncogenic RET and of the MTC cell line TT in nude mice, AST-487 dose dependently inhibited tumor growth. It also demonstrates antiproliferative effects on primary cells from acute myelocytic leukemia patients and on cell lines expressing FLT3-ITD or FLT3 kinase domain point mutants.[Cayman Chemical]
Biological Activity
ast487 is an inhibitor of ret kinase with ic50 value of 0.88μm [1].ast487 belongs to the n,n’-diphenyl urea class. it inhibit the activity of ret kinase as well as many other kinases( such as kdr, flt-3 and c-kit) in vitro. in the cell assay, the inhibition effect of ast487 is displayed both in pc-ret/ptc3 cells and tt cells, which harbor an endogenous activating point mutation of ret (retc634w). ast487 decreases ret autophosphorylation and activation of plcγ and erk with a dose-dependent manner. additionally, ast487 is also found to inhibit the growth of human thyroid cancer cell lines with ret, but not braf mutations. it supports the selectivity of ast487 for ret. in vivo assay shows that ast487 causes significant reductions in the size of nih3t3-retc634w xenografts with doses >30 mg/kg/d and oral administration of ast487 at 50 or 30 mg/kg/d decreases mean tumor volume in mice [1].
References
[1] nagako akeno-stuart, michelle croyle, jeffrey a. knauf, et al. the ret kinase inhibitor nvp-ast487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. cancer res. 2007, 67:6956-6964.