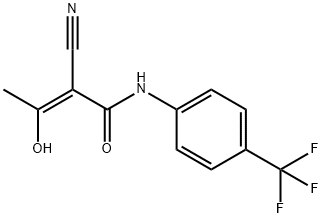
Description
In September 2012, the US FDA approved teriflunomide (also referred
to as HMR-1726) as a therapy for patients with relapsing forms of multiple
sclerosis (MS). Teriflunomide is the second approved oral treatment option
for MS, after Gilenya? which was approved in 2010. Teriflunomide, which is the active metabolite of leflunomide (a marketed
drug for the treatment of rheumatoid and psoriatic arthritis), is a non?competitive and selective inhibitor of dihydroorotate dehydrogenase
(DHODH), the rate-limiting enzyme in the de novo synthesis of pyrimidines.
Although the net effect of inhibition of DHODH by teriflunomide and its
therapeutic effect in MS are not clear, it is hypothesized that by inhibiting de novo pyrimidine synthesis, the effector functions of activated lymphocytes
are suppressed, thus dampening the effect of an overactive immune system.
Description
A-771726 is an active metabolite of the prodrug leflunomide that inhibits dihydroorotate dehydrogenase (DHODH; IC
50 = 0.23 μM). A-771726 inhibits the production of prostaglandin E
2 (PGE
2; ) in TNF-α- or IL-1α-stimulated isolated human synoviocytes (IC
50s = 7 and 3 μM, respectively). It inhibits the proliferation of isolated human peripheral blood mononuclear cells (PBMCs) when used at concentrations of 25 and 100 μM. A-771726 (3 and 10 mg/kg) delays disease onset and decreases neurological deficits in a rat model of experimental autoimmune encephalomyelitis (EAE) induced by complete Freund’s adjuvant (CFA) and
M. tuberculosis. Formulations containing teriflunomide have been used in the treatment of multiple sclerosis.
Chemical Properties
White Solid
Originator
Genzyme (United States)
Uses
A-771726 is the active metabolite of leflunomide, a prodrug approved by the FDA for treatment of rheumatoid arthritis. A-771726 reversibly inhibits dihydroorotate dehydrogenase, the rate-limiting step in the de novo synthesis of pyrimidines. It prevents activated lymphocytes from accumulating sufficient pyrimidines to support DNA synthesis (IC50s = 0.09, 3.5, and 12.5 μM for rat, mouse, and human, respectively). At higher doses, A-771726 inhibits tyrosine kinases responsible for early T cell and B cell signaling in the G0/G1 phase of the cell cycle. A-771726 has also been shown to inhibit the production of prostaglandin E2 in synoviocytes activated by TNF-α and IL-1α (IC50s = 7 and 3 μM, respectively) as well as inhibit MMP-1 and IL-6 production at concentrations >10 μM.[Cayman Chemical]
Uses
Teriflunomide has been used as a dihydroorotate dehydrogenase (DHODH) inhibitor.
Uses
The active metabolite of Leflunomide, a potent disease-modifying antirheumatic drug used in the treatment of rheumatoid arthritis. LEF-M interferes with dendritic cell function.
Definition
ChEBI: An enamide obtained by formal condensation of the carboxy group of (2Z)-2-cyano-3-hydroxybut-2-enoic acid with the anilino group of 4-(trifluoromethyl)aniline. Used for the treatment of relapsing forms of multiple sclerosis and rheumatoid
arthritis.
General Description
Teriflunomide, marketed under the trade name Aubagio, is the active metabolite of leflunomide, an immunosuppressive disease-modifying antirheumatic drug, used in active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. An analytical reference standard for use in LC/MS or GC/MS applications including clinical and diagnostic testing such as therapeutic drug monitoring assays.
Biochem/physiol Actions
Teriflunomide is an orally available anti-inflammatory immunomodulator. It blocks the activity of dihydroorotate dehydrogenase, preventing pyrimidine synthesis and T and B cell proliferation and function. Teriflunomide has been used to treat rheumatoid arthritis and was recently approved for multiple sclerosis.
References
[1] yao h w, li j, chen j q, et al. a 771726, the active metabolite of leflunomide, inhibits tnf-α and il-1 from kupffer cells[j]. inflammation, 2004, 28(2): 97-103.
[2] breedveld f c, dayer j m. leflunomide: mode of action in the treatment of rheumatoid arthritis[j]. annals of the rheumatic diseases, 2000, 59(11): 841-849.
[3] burger d, begué‐pastor n, benavent s, et al. the active metabolite of leflunomide, a77 1726, inhibits the production of prostaglandin e2, matrix metalloproteinase 1 and interleukin 6 in human fibroblast‐like synoviocytes[j]. rheumatology, 2003, 42(1): 89-96.
[4] davis j p, cain g a, pitts w j, et al. the immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase[j]. biochemistry, 1996, 35(4): 1270-1273.