Biological Activity
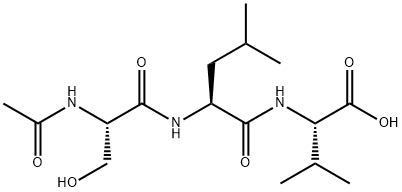
fas c- terminal tripeptide,(c16h29n3o6), a tri-peptide with the sequence ac-ser-leu-val-oh, it’s the c-terminal tripeptide of fas, mw= 359.4. fas (apo-1/cd95) is a cell surface receptor, which is a member of the tumor necrosis factor receptor (tnfr) superfamily, the fas receptor is a death receptor on the surface of cells that leads to programmed cell death (apoptosis). it is one of two apoptosis pathways. fas forms the death-inducing signaling complex (disc) upon ligand binding. upon ensuing death domain (dd) aggregation, the receptor complex is internalized via the cellular endosomal machinery. this allows the adaptor molecule fadd to bind the death domain of fas through its own death domain. fadd also contains a death effector domain (ded) near its amino terminus,which facilitates binding to the ded of fadd-like interleukin-1 beta-converting enzyme (flice), more commonly referred to as caspase-8. flice can then self-activate through proteolytic cleavage into p10 and p18 subunits, two each of which form the active heterotetramer enzyme. active caspase-8 is then released from the disc into the cytosol, where it cleaves other effector caspases, eventually leading to dna degradation, membrane blebbing, and other hallmarks of apoptosis.the c-terminal tripeptide (ac-ser-leu-val-oh) of fas was necessary and sufficient both for binding to the third pdz domain of fap-1 and for inhibiting fas/fap-1 binding.figure1 formula of fas c- terminal tripeptidefigure2 signaling pathway of fas
References
1. Lichter P, Walczak H, Weitz S, Behrmann I, Krammer PH (September 1992). "The human APO-1 (APT) antigen maps to 10q23, a region that is syntenic with mouse chromosome 19". Genomics 14 (1): 179–80.2. Inazawa J, Itoh N, Abe T, Nagata S (November 1992). "Assignment of the human Fas antigen gene (Fas) to 10q24.1". Genomics 14 (3): 821–2.3. Huang B, et al. (1996). "NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain". Nature 384 (6610): 638–41.4. Eberstadt M, et al. (1998). "NMR structure and mutagenesis of the FADD (Mort1) death-effector domain". Nature 392 (6679): 941–5.