Manufacturing Process
To a stirred solution of 3-trifluoromethyl-α-(p-toluenesulfonyloxyimino)
benzylcyanide in methanol containing methyl thioglycolate was added
dropwise over a 30 min period triethylamine. The reaction mixture was stirred
at room temperature for 4 h following complete addition, and then was cooled
to 0°C and filtered. The precipitate which was collected was recrystallized
from hexane and ethyl acetate to provide methyl 3-(3-trifluoromethylphenyl)-
4-amino-5-isothiazolecarboxylate, melting point 94°-95°C.
A solution of methyl 3-(3-trifluoromethylphenyl)-4-amino-5-
isothiazolecarboxylate and potassium hydroxide in methanol was heated at
reflux for 6 h. The reaction mixture was then poured into ice containing 12 N
aqueous hydrochloric acid. The free acid precipitated from the acidic solution,
and after all of the ice had melted, the aqueous acidic mixture was filtered.
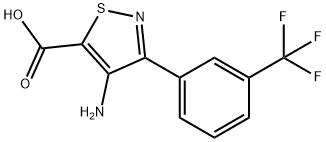
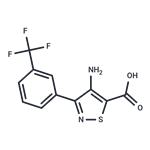
The precipitate was crystallized from ethanol and water to provide 3-(3-
trifluoromethylphenyl)-4-amino-5-isothiazolecarboxylic acid, melting point
179°-180°C. Yield 80%.