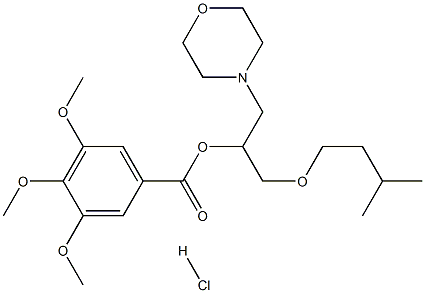
Manufacturing Process
To a mixture of 176 g (2 M) of isoamyl alcohol and 4 ml of a 10% solution of
BF3 in anhydrous ether, were added, with stirring, 278 g (3 M) of
epichlorohydrin while maintaining the temperature at approximately 45°C.
After the addition, the reaction was maintained for an additional hour at 60°C.
It was then cooled and a solution of 160 g of NaOH in pellets in 200 ml H2O
was added while maintaining the temperature at approximately 15°C. Stirring
was continued for an additional 2 hours, the NaCl formed was filtered and the
organic phase decanted. Vacuum fractionation gave 142 g of 3-isoamyloxy-
1,2-epoxypropane. Boiling point 67-68°C.
To a solution of 115.2 g (0.8 M) of 3-isoamyloxy-1,2-epoxypropane in 200 ml
of absolute ethanol were added 76 g of morpholine. The temperature rose to
approximately 40°C. Heating under reflux was continued for one additional
hour, the solvent was distilled and the reaction mixture vacuum fractionated
to obtain 166 g of 4-[3-isoamyloxy-2-hydroxy]propyltetrahydro-1,4-oxazine.
Boiling point 163°C, nD
21 = 1.4620, yield=90%.
In a 2 liter three-neck flask, provided with a tight-fitting stirrer and a reflux
condenser, were charged: 115.5 g (0.5 M) of 4-[3-isoamyloxy-2-
hydroxy]propyl tetrahydro-1,4-oxazine in 750 ml of anhydrous benzene, 50.5
g of triethylamine and 115.25 g of (3,4,5-trimethoxy)benzoyl chloride. The
mixture was slowly brought to reflux, which was maintained for 4 hours. After
cooling, it was filtered, the solvent was stripped off under vacuum, the residue
taken up in 4 N HCl (in the cold) and the aqueous solution washed with ether.
The aqueous solution was then treated with sodium carbonate and the oil
formed extracted with the ether. It was dried over anhydrous sodium sulfate
and the solvent tripped off to obtain a highly viscous oily residue, which was
taken up in 600 ml of ethyl acetate; the required quantity of absolute ethanol,
with 30% HCl gas, was then added, affording the hydrochloride. After
standing for several hours in the ice-box, it was filtered, and after
recrystallization from anhydrous isopropyl alcohol and drying under vacuum at
60°C to constant weight, 155 g of (3,4,5-trimethoxy)benzoyl chloride were
obtained. Yield=67%, melting point 145°C.