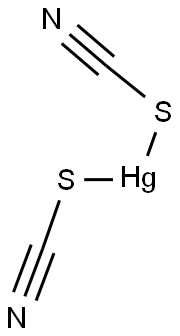
Description
Mercury thiocyanate is a white, odorless powder. Molecular weight= 316.79; Freezing/Melting point=about 165℃ (decomposes). Slightly soluble in cold water.
Chemical Properties
Mercuric thiocyanate is an inorganic chemical substance. It is a stable solid at room temperature, and depending upon the purity, it appears as odourless white crystalline powder or grey. It is insoluble in water and denser than water and sinks in water. The solubility in alcohol and boiling H2O
and in KSCN solution is higher, in ether lower. Decomposes with
swelling on heating to 165°C. On decomposition, mercuric thiocyanate releases hazardous substances such as cyanide vapours, vapours of mercury, oxides of nitrogen (NO, NO2), and oxides of sulphur (SO2, SO3). Mercury thiocyanate has limited uses in chemical synthesis.
Chemical Properties
Mercury thiocyanate is a white, odorless
powder.
Uses
Mercury(II) thiocyanate is used as a precursor to potassium tris(thiocyanato)mercurate(II) and cesium tris(thiocyanato)mercurate(II). It is also used in the determination of chloride ions in water by UV-visible spectroscopy. Further, it acts as a catalyst for the addition of thiocyanic acid to alkynes.
Uses
For Pharaoh's serpents (fireworks); intensifier in photography.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
MERCURIC THIOCYANATE decomposes into its elements at about 165°C. Burns readily in air to generate a coil of cohesive ash resembling a serpent (hence used in a firework: Pharaoh's serpents). Swells up to many times its original volume if heated [USCG, 1999]. Soluble in dilute hydrochloric acid [Merck]. Incompatible with strong oxidizing agents.
Hazard
Highly toxic by ingestion, inhalation, and
skin absorption.
Health Hazard
Mercuric thiocyanate causes severe eye and skin irritation with possible burns and causes digestive and respiratory tract irritation with possible burns. It may impair fertility, may cause harm to the unborn child, is harmful if inhaled, may cause allergic skin reaction, may cause kidney damage, may cause CNS effects, is light sensitive, and is a severe marine pollutant. Contact with acids liberates very toxic gas. The target organs include kidneys, CNS, reproductive system, eyes, and skin.
reaction suitability
core: mercury
reagent type: catalyst
Safety Profile
A poison by ingestion
and intraperitoneal routes. Moderately toxic
by skin contact. Thermally unstable and
decomposition may be vigorous. When
heated to decomposition it emits very toxic
fumes of Hg, NOx, SOx, and CN-. See also
MERCURY COMPOUNDS and
CYANATES.
Synthesis
A Hg(NO3)2
solution, acidified with a few drops of HNO3,
is treated with the stoichiometric amount of KSCN solution. The
resultant crystalline precipitate is suction-filtered and washed
with H2O. The product may be recrystallized from hot H2O or
alcohol. Yield 80%.
Hg(NO3)2 + 2 KSCN = Hg(SCN)2 + 2KNO3
Potential Exposure
Mercury thiocyanate is used in
photography and fireworks.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Antidotes and special procedures for medical personnel: The drug NAP (n-acetyl penicillamine) has been used to treat mercury poisoning, with mixed success. Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has been used to treat toxic symptoms of certain heavy metals poisoning including mercury. Although BAL is reported to have a large margin of safety, caution must be exercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with 1-ephedrine sulfate (CAS: 134-72-5).
storage
Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area away from light, heat, and acids, including fumes.
Shipping
UN1646 Mercury thiocyanate, Hazard Class:
6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Recrystallise it from H2O, and it can give various crystal forms depending on conditions. Its solubility in H2O is 0.069% at 25o, but is more soluble at higher temperatures. It decomposes to Hg above 165o. Poisonous. [Mason & Forgeng J Phys Chem 35 1121 1931, Birckenbach & Kolb Chem Ber 68 919 1935.]
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Mercury thiocyanate is
sensitive to heat; expands to many times its original volume
and then decomposes at freezing/melting point forming
toxic fumes of sulfur oxides, mercury cyanide, and nitrogen
oxides. Contact with acid or acid fumes causes release
of toxic mercury and cyanide vapors. Incompatible with
chlorine, reducing agents such as hydrides, sulfides
Waste Disposal
Small amounts may be
destroyed by alkaline hydrolysis. Admixture with alkali
can be followed by soil burial. Larger quantities can be
disposed of by incineration in admixture with acetone or
xylene and using effluent gas scrubbing. Do not reuse
empty container; proper disposal required.