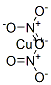Description
Copper Nitrate consists of the elements Copper, Nitrogen, and Oxygen. Copper is a metal with good electrical and thermal conductivity. It is present in group 11 of the periodic table. Copper is represented by the symbol Cu, and its atomic number is 29. Nitrogen is a colorless, tasteless, odorless gas and the most plentiful element in Earth’s atmosphere. It is present in group 15 of the periodic table and is represented with the symbol N. Oxygen is a highly reactive nonmetal present in group 16 of the periodic table. Its atomic number is 8 and is represented by the symbol O. Anhydrous copper nitrate forms dark blue-green gems and sublimes at 150-200 °C in a vacuum. Copper nitrate additionally happens as five different hydrates; the most widely recognized ones are copper II nitrate, hemipentahydrate, hemipentahydrate, and Copper II nitrate trihydrate.

Uses
Copper nitrates find a variety of applications, the main one being its conversion to copper(II) oxide, which is used as a catalyst for various processes in organic chemistry. Its solutions are used in textiles and polishing agents for other metals. Copper nitrates are found in some pyrotechnics. It is often used in school laboratories to demonstrate chemical voltaic cell reactions. It is a component in some ceramic glazes and metal patinas. Copper nitrate, in combination with acetic anhydride, is an effective reagent for nitration of aromatic compounds, known as Menke nitration.