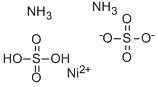Chemical Properties
dark green crystals
Chemical Properties
Nickel ammonium sulfate is a green, odorless
powder.
Uses
Nickel electrolyte for electroplating.
Definition
ChEBI: A nickel coordination entity comprising ammonium, nickel and sulfate in which the ratio of ammonium to iron(2+) to sulfate ions is 2:1:2.
General Description
NICKEL AMMONIUM SULFATE is a green crystalline solid. Mildly toxic, carcinogenic. When heated to decomposition NICKEL AMMONIUM SULFATE emits highly toxic fumes of metallic nickel, oxides of sulfur, and oxides of nitrogen (Lewis, 3rd ed., 1993, p. 910). The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. NICKEL AMMONIUM SULFATE is used for electroplating nickel.
Air & Water Reactions
Water soluble. Forms an acidic aqueous solution.
Reactivity Profile
NICKEL AMMONIUM SULFATE is a "double salt" that is a weak oxidizing agent. Gives an acidic solution when dissolved in water.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion causes vomiting. Contact with eyes causes irritation. Contact with skin may cause dermatitis.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen may be formed in fire.
Safety Profile
Confirmed human
carcinogen. Poison by ingestion. When
heated to decomposition it emits toxic
fumes of NOx, SOx, and Ni.
Potential Exposure
This material is used in electroplating.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous
material, Technical Name Required.
Purification Methods
Crystallise this salt from water (3mL/g) on cooling from 90o to 0o.
Incompatibilities
Forms an acidic solution with water.
A weak oxidizer; keep away from combustible materials,
reducing agents, including hydrides. Possible violent
reaction with strong acids. Incompatible with nickel nitrate,
sulfur, selenium, wood, organics, and other combustibles.