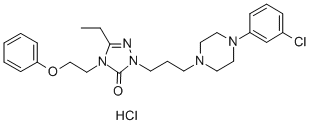
Description
Nefazodone, a second generation trazodone type antidepressant, was launched in Canada. Nefazodone acts as both a potent 5-HT
2 receptor antagonist
and as a serotonin (5-HT) reuptake inhibitor. This combination effect appears to
enhance 5-HT
IA-mediated neurotransmission. Distinct from the first generation
agents such as tricyclic antidepressants, nefazodone has very selective
serotonergic effects with negligible affinity for cholinergic and histamine receptors,
and low affinity for a
1 -adrenergic receptors. Therefore, it has a superior toxicity
profile that does not produce agitation, insomnia, and lacks cardiac side effects.
Chemical Properties
White Solid
Originator
Bristol-Myers Squibb (U.S.A.)
Uses
An antidepressant acts by modifying serotonin transmission. Mixed 5-HT2A serotonin receptor antagonist/serotonin uptake inhibitor
Uses
A selective serotonin 5-HT2 receptor antagonist. A novel antidepressant that shows no cardiac toxicity or anticholinergic activity common with tricyclic antidepressants.
Definition
ChEBI: Nefazodone hydrochloride is a hydrochloride. It contains a nefazodone.
Manufacturing Process
Manufacturing process for Moxifloxacin hydrochloride includes these steps as follows: 1. synthesis of 1-(3-Chloropropyl)-4-(3-chlorophenyl)piperazine hydrochloride, 1-(3-Chlorophenyl)-4-(hydrazinopropyl)piperazine. 2.Phenoxypropionic acid (249.0 g, 1.50 mol) is dissolved in four equivalents
of thionyl chloride (438.0 ml, 6.0 mol) and heated to reflux until the HCl
evolution has ceased. The solution is then cooled to room temperature and
concentrated under reduced pressure to give 281.0 g (100% yield) of
phenoxypropionyl chloride as a brown oil which solidifies on cooling 3.Phenoxypropionyl chloride (9.23 g, 0.05 mol) is dissolved in 100 ml
acetone and cooled with an ice bath as sodium azide (3.6 g, 0.055 mol) in 10
ml water is added dropwise. After addition is completed, the reaction mixture
is warmed to room temperature and stirred for 30 minutes. The solution is
decanted and concentrated. The residue is dissolved in 100 ml ether and
washed with saturated sodium bicarbonate and brine. The organic phase is
separated, dried (MgSO4) and concentrated to give 6.52 g (68.0% yield) of
phenoxypropionyl azide as a yellow oil which solidifies on cooling. 4.Synthesis of Ethyl phenoxypropionate, Phenoxypropionyl hydrazide, Phenoxyethyl isocyanate, 2-3-(4-[3-Chlorophenyl]-1-piperazinyl)propyl]-4-(2-phenoxyethyl)-
semicarbazide), and 2-[3-4-(3-Chlorophenyl)-1-piperazinyl]-propyl]-5-ethyl-4-(2-phenoxyethyl)-
2H-1,2,4-triazol-3(4H)-one monohydrochloride (Nefazodone
monohydrochloride).
brand name
Serzone (Bristol-Myers Squibb).
Therapeutic Function
Antidepressant
General Description
Nefazodone, formerly sold under the brand names Serzone, Dutonin, and Nefadar, is an antidepressant that has been discontinued due to the incidence of severe liver damage. This certified Snap-N-Spike
; solution is suitable for use as a starting material in calibrators and controls for numerous GC/MS or LC/MS applications in forensic analysis, clinical toxicology and urine drug testing.
Biological Activity
Serotonin 5-HT 2A receptor antagonist (K i = 5.8 nM) and inhibitor of serotonin and noradrenalin uptake (IC 50 values are 290 and 300 nM respectively). Displays no activity at 5-HT 1B and 5-HT 1D receptors. Active in models predictive of antidepressant potential.
Biochem/physiol Actions
Novel antidepressant; mixed 5-HT2A serotonin receptor antagonist/serotonin uptake inhibitor.