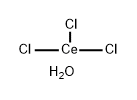
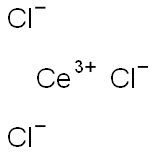Chemical Properties
Cerium(III) chloride is a white powder and is used for incandescent gas mantles, spectrography and polymerization of olefins.

Cerium(III) chloride is mainly used as catalyst for petroleum,automobile exhaust catalyst , also used as a raw material for the production of cerium metal and other cerium compounds.
Physical properties
White, very fine powder; hexagonal crystal system; heptahydrate is yellow orthogonal crystal and hygroscopic; density of anhydrous salt 3.97 g/cm
3; melts at 817°C; vaporizes at 1,727°C; heptahydrate begins to lose water above 90°C and becomes anhydrous at about 230°C; soluble in water and alcohol; hexahydrate has greater solubility in these solvents.
Uses
Cerium(III) chloride is used in incandescent gas mantles, spectrography and polymerization of olefins. It acts as a Lewis acid and used in Friedel-Crafts alkylation and acylation reactions in organic synthesis. It is used as a precursor for the preparation of other cerium salts, such as cerium(III) trifluoromethanesulfonate.
Production Methods
Cerium(III) chloride is prepared by the reaction of hydrochloric acid with a cerium salt, such as cerium hydroxide or carbonate, followed by crystallization;
Ce(OH)
3 + 3HCl → CeCl
3 + 3H
2O
Ce
2(CO
3)
3 + 6HCl → 2CeCl
3 + 3CO
2 + 3H
2O.
Flammability and Explosibility
Non flammable
reaction suitability
reagent type: catalyst
core: cerium
Safety Profile
Poison by intravenous,
intraperitoneal, and subcutaneous routes.
Moderately toxic by ingestion. See also
CERIUM COMPOUNDS. When heated to
decomposition it emits toxic fumes of Cl-