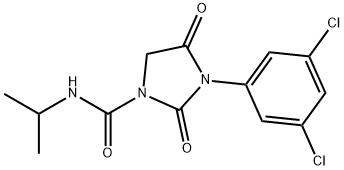
3-(3,5-Dichlorphenyl)-2,4-dioxo-N-isopropylimidazolidin-1-carboxamid
Bezeichnung:3-(3,5-Dichlorphenyl)-2,4-dioxo-N-isopropylimidazolidin-1-carboxamid
CAS-Nr36734-19-7
Englisch Name:Iprodione
CBNumberCB7131064
SummenformelC13H13Cl2N3O3
Molgewicht330.17
MOL-Datei36734-19-7.mol
Synonyma
3-(3,5-Dichlorphenyl)-2,4-dioxo-N-isopropylimidazolidin-1-carboxamid
3-(3,5-Dichlorphenyl)-2,4-dioxo-N-isopropylimidazolidin-1-carboxamid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 130-134 °C(lit.) |
| Dichte | 1.6223 (rough estimate) |
| Dampfdruck | 5 x 10-7 Pa (25 °C) |
| Brechungsindex | 1.6140 (estimate) |
| Flammpunkt | 2 °C |
| storage temp. | Sealed in dry,Room Temperature |
| Löslichkeit | DMSO: 100 mg/mL (302.87 mM) |
| pka | 9.19±0.20(Predicted) |
| Aggregatzustand | Solid |
| Farbe | White to off-white |
| Wasserlöslichkeit | 0.0013 g/100 mL |
| Merck | 13,5096 |
| BRN | 895003 |
| LogP | 3.000 |
| CAS Datenbank | 36734-19-7(CAS DataBase Reference) |
| NIST chemische Informationen | Iprodione(36734-19-7) |
| EPA chemische Informationen | Iprodione (36734-19-7) |
| Kennzeichnung gefährlicher | Xn,N,F |
| R-Sätze: | 40-50/53-36-20/21/22-11 |
| S-Sätze: | 36/37-60-61-36-26-16 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS-Nr. | NI8870000 |
| HS Code | 29332900 |
| Giftige Stoffe Daten | 36734-19-7(Hazardous Substances Data) |
| Toxizität | LD50 in mice, rats (g/kg): 4, 3.5 orally (Lacroix, 1980) |


