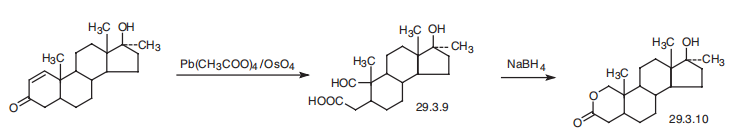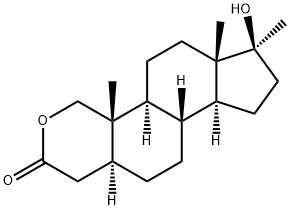
Oxandrolon
Bezeichnung:Oxandrolon
CAS-Nr53-39-4
Englisch Name:Oxandrolone
CBNumberCB6112482
SummenformelC19H30O3
Molgewicht306.44
MOL-Datei53-39-4.mol
Synonyma
Oxandrolon
Oxandrolon physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 235-238°C |
| alpha | D25 -23° (c = approx 1% in chloroform) |
| Siedepunkt | 387.04°C (rough estimate) |
| Dichte | 1.0829 (rough estimate) |
| Brechungsindex | 1.4200 (estimate) |
| storage temp. | 2-8°C |
| Löslichkeit | DMSO: soluble2mg/mL (clear solution; warmed) |
| Aggregatzustand | powder |
| pka | 15.15±0.60(Predicted) |
| Farbe | white to beige |
| Optische Aktivität | [α]/D -19 to -26°, c = 1 (CDCl3) |
| Merck | 6921 |
| InChIKey | QSLJIVKCVHQPLV-PEMPUTJUSA-N |
| CAS Datenbank | 53-39-4(CAS DataBase Reference) |
| NIST chemische Informationen | Oxandrolone(53-39-4) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 60-63-20/21/22 |
| S-Sätze: | 22-36/37/39-45 |
| WGK Germany | 3 |
| RTECS-Nr. | RN6800700 |
| HS Code | 2937290000 |
| Giftige Stoffe Daten | 53-39-4(Hazardous Substances Data) |
| Toxizität | LD50 oral in rabbit: > 5gm/kg |