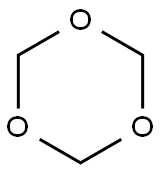
1,3,5-Trioxan
Bezeichnung:1,3,5-Trioxan
CAS-Nr110-88-3
Englisch Name:1,3,5-trioxane
CBNumberCB5763739
SummenformelC3H6O3
Molgewicht90.08
MOL-Datei110-88-3.mol
Synonyma
1,3,5-Trioxan
1,3,5-Trioxan physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 59-62 °C(lit.) |
| Siedepunkt | 112-115 °C |
| Dichte | 1.17 |
| Dampfdruck | 7.5 hPa (20 °C) |
| Brechungsindex | 1.4168 (estimate) |
| Flammpunkt | 113 °F |
| storage temp. | Store below +30°C. |
| Löslichkeit | 221g/l |
| Aggregatzustand | Crystals or Crystalline Flakes |
| Farbe | Colorless to white |
| Explosionsgrenze | 3.6-28.7%(V) |
| Wasserlöslichkeit | 221 g/L (25 ºC) |
| Sublimation | 115 ºC |
| Merck | 14,9734 |
| BRN | 102769 |
| InChIKey | BGJSXRVXTHVRSN-UHFFFAOYSA-N |
| CAS Datenbank | 110-88-3(CAS DataBase Reference) |
| NIST chemische Informationen | 1,3,5-Trioxane(110-88-3) |
| Kennzeichnung gefährlicher | F,Xn |
| R-Sätze: | 11-37-63 |
| S-Sätze: | 36/37-46 |
| RIDADR | UN 1325 4.1/PG 2 |
| WGK Germany | 1 |
| RTECS-Nr. | YK0350000 |
| Selbstentzündungstemperatur | 777 °F |
| TSCA | Yes |
| HS Code | 2912 50 00 |
| HazardClass | 4.1 |
| PackingGroup | III |
| Giftige Stoffe Daten | 110-88-3(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 8190 mg/kg LD50 dermal Rabbit > 3980 mg/kg |



