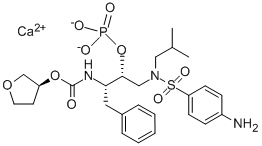
Fosamprenavir calcium
Bezeichnung:Fosamprenavir calcium
CAS-Nr226700-81-8
Englisch Name:Fosamprenavir calcium
CBNumberCB5496914
SummenformelC25H34CaN3O9PS
Molgewicht623.668921
MOL-Datei226700-81-8.mol
Fosamprenavir calcium physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 282-2840C |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| Löslichkeit | Aqueous Acid (Slightly), DMF (Slightly) |
| Aggregatzustand | Solid |
| Farbe | White to Off-White |
| BCS Class | 2 |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)


-
Alarmwort
Warnung
-
Gefahrenhinweise
H315:Verursacht Hautreizungen.
H361:Kann vermutlich die Fruchtbarkeit beeinträchtigen oder das Kind im Mutterleib schädigen.
-
Sicherheit
P201:Vor Gebrauch besondere Anweisungen einholen.
P202:Vor Gebrauch alle Sicherheitshinweise lesen und verstehen.
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P281:Vorgeschriebene persönliche Schutzausrüstung verwenden.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P308+P313:BEI Exposition oder falls betroffen: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
P321:Besondere Behandlung
P332+P313:Bei Hautreizung: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
P362:Kontaminierte Kleidung ausziehen und vor erneutem Tragen waschen.
P405:Unter Verschluss aufbewahren.
P501:Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen.
Fosamprenavir calcium Chemische Eigenschaften,Einsatz,Produktion Methoden
-
Beschreibung
Fosamprenavir, a prodrug of the HIV protease inhibitor amprenavir, is indicated for the oral treatment of HIV infection in adults in combination with other antiretroviral agents. Although amprenavir has excellent antiviral potency and good tolerability, its watersolubility is poor (0.04 mg/ml). As a result, the formulation of the agent includes a high percentage of organic excipients to facilitate gastric dissolution, which limits the amount of active drug that can be formulated per capsule. Fosamprenavir is a highly soluble phosphate ester of amprenavir. It allows more convenient dosing and reduction in pill counts as compared to amprenavir. Fosamprenavir is readily prepared in two steps starting from a key intermediate used in the synthesis of amprenavir, by phosphorylating a hydroxyl group and subsequently reducing a p-nitrophenyl to a p-aminophenyl group. Fosamprenavir has little or no antiviral activity in vitro. After oral administration, it is rapidly and almost completely hydrolyzed by phosphatases in the gut epithelium to amprenavir prior to reaching systemic circulation. The time to reach peak plasma concentration of amprenavir is approximately 2.5 h and the plasma elimination half-life is approximately 7.7 h. Amprenavir is metabolized in the liver by CYP3A4 and >90% of the dose is excreted as metabolites in urine and feces. In most patients, fosamprenavir is administered at daily doses of 700–1400 mg in conjunction with ritonavir. Monotherapy with fosamprenavir is only recommended in antiretroviral therapy-na?ve patients and the dosing regimen is 1400 mg twice daily. The most common adverse events experienced with fosamprenavir are diarrhea, nausea, vomiting, headache and rash. -
Beschreibung
Fosamprenavir (calcium salt) is an orally bioavailable prodrug of the HIV-1 protease inhibitor amprenavir . Fosamprenavir has improved solubility compared with amprenavir, and its pharmacokinetics, either during fasting or with a low- or high-fat meal, suggest that it could be effective using fewer tablets and a less complex dosing schedule than other HIV treatments. Formulations containing fosamprenavir are used for adult and pediatric patients with HIV infection, especially as an initial antiretroviral therapy. -
Chemische Eigenschaften
White Microcrystalline Needles -
Originator
Vertex (US) -
Verwenden
HIV protease inhibitor; water soluble prodrug of amprenavir. -
Verwenden
Protease inhibitor, anti-HIV agent -
Trademarks
Lexiva (GlaxoSmithKline). -
Allgemeine Beschreibung
Fosamprenavir is used in combination with otherHIV drugs in adult patients. Like the other PIs, this compoundis a prodrug that produces the active drug uponhydrolysis. In this case, the active drug is amprenavir, apeptidomimetic transition state inhibitor. Fosamprenaviris typically administered in combination with RTinhibitors. -
Clinical Use
Fosamprenavir calcium has been approved for the treatment of HIV in adults when used in combination with other anti-HIV drugs. It is a prodrug that, on hydrolysis by serum phosphatases, gives rise to amprenavir, which is a peptidomimetic transition-state inhibitor that targets HIV-1 protease and reduces the viral replication and, thus, the infectiousness of HIV-1. It is commonly administered in combination with RT inhibitors to produce excellent efficacy in patients with AIDS. The drug is administered as two 700 mg tablets twice daily or, in combination with ritonavir, can be given as two 700 mg tables once daily or one 700 mg tablet twice daily. As a result, formaprenavir lowers the "pill burden" in patients with AIDS. -
Synthese
The synthesis of fosamprenavir (X) started with a known amino alcohol 91. N,N-Dibenzyl-L-phenylalaninal (87) was prepared by reduction of L-phenylalanine (86) to L-phenylalaninol followed by N,N-dibenzylation and oxidation to the aldehyde 87 using pyridine-sulfur trioxide complex at room temperature. A large excess of lithium shot was stirred in a solution of aldehyde 87 and bromochloromethane in THF at -65??C. The reaction mixture was subsequently allowed to warm up to room temperature to provide the diastereomeric epoxide mixture (6:1) which was quenched with 6N aqueous HCl and set standing overnight to provide the salt precipitate. Recrystallization from methanol gave optically pure dibenzylaminochlorohydrin hydrochloride (88) in 38- 45% yield. Hydrogenolysis under standard conditions gave deprotected aminochlorohydrin hydrochloride 89 as a crystalline white solid. Conversion to desired N-Bocepoxide 90 was accomplished by the introduction of the Boc group followed by cyclization. N-Boc-epoxide 90 was then converted to amino alcohol 91 by refluxing with isobutylamine in EtOH. Treatment of the amino alcohol91 with p-nitrobenzene sulphonyl chloride in toluene at 80??C followed by acid hydrolysis of the Boc group furnished sulphonamide 93 in 73% yield. The carbamate 95 was prepared by refluxing 93 with (S)-tetrahydrofuryl imidazole carboxylate (94) in EtOAc. Treatment of the sulphonamide 95 with POCl3 followed by aqueous HCl hydrolysis provided the phosphate intermediate, which was then reduced by hydrogenation and converted to fosamprenavir calcium salt X in a one-pot process in 92% yield.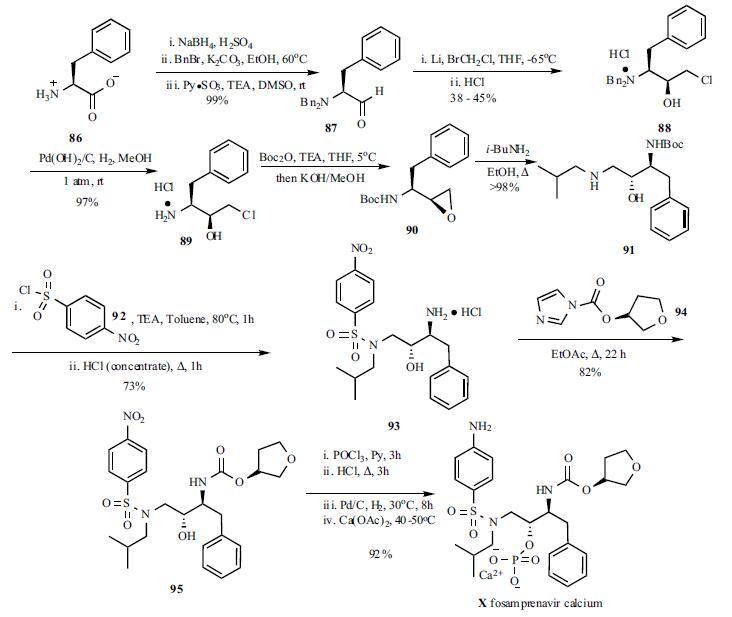
-
Arzneimittelwechselwirkung
Potentially hazardous interactions with other drugs Anti-arrhythmics: possibly increased concentration of amiodarone, flecainide, lidocaine and propafenone (increased risk of ventricular arrhythmias) - avoid. Antibacterials: increases concentration of rifabutin - reduce rifabutin dose; concentration significantly reduced by rifampicin - avoid; avoid with telithromycin in severe renal and hepatic impairment. Anticoagulants: avoid with apixaban and rivaroxaban. Antidepressants: concentration reduced by St John’s wort - avoid. Antimalarials: use artemether/lumefantrine with caution; possibly increases quinine concentration.
Antipsychotics: possibly inhibits aripiprazole metabolism - reduce aripiprazole dose; possibly increases quetiapine concentration - avoid; possibly increases pimozide concentration (increased risk of ventricular arrhythmias) - avoid.
Antivirals: avoid with boceprevir, raltegravir and telaprevir; concentration of dolutegravir reduced; concentration increased by etravirine, consider reducing fosamprenavir dose; concentration reduced by lopinavir, maraviroc and tipranavir, effect on lopinavir unpredictable - avoid, avoid with maraviroc; concentration possibly reduced by nevirapine; avoid with raltegravir.
Anxiolytics and hypnotics: increased risk of prolonged sedation and respiratory depression with midazolam - avoid with oral midazolam.
Avanafil: concentration of avanafil possibly increased.
Cytotoxics: possibly increases concentration of bosutinib and ibrutinib, avoid or consider reducing bosutinib and ibrutinib dose.
Ergot alkaloids: increased risk of ergotism - avoid.
Immunosuppressants: monitor ciclosporin,
tacrolimus and sirolimus levels. Lomitapide: avoid concomitant use.
Orlistat: absorption possibly reduced by orlistat.
Ranolazine: possibly increases ranolazine concentration - avoid. Statins: possibly increased risk of myopathy with atorvastatin; possibly increased myopathy with simvastatin and rosuvastatin - avoid. -
Stoffwechsel
Fosamprenavir is rapidly and almost completely hydrolysed to amprenavir and inorganic phosphate as it is absorbed through the gut epithelium, following oral administration. The primary route of metabolism of amprenavir is via the cytochrome P450 3A4 enzyme. The primary route of elimination of amprenavir is via hepatic metabolism with less than 1% excreted unchanged in the urine and no detectable amprenavir in faeces. Metabolites account for approximately 14% of the administered amprenavir dose in the urine, and approximately 75% in the faeces. -
Einzelnachweise
[1]. wire, m.b., et al., pharmacokinetics and safety of gw433908 and ritonavir, with and without efavirenz, in healthy volunteers. aids, 2004. 18(6): p. 897-907.
[2]. hamada, y., et al., high incidence of renal stones among hiv-infected patients on ritonavir-boosted atazanavir than in those receiving other protease inhibitor-containing antiretroviral therapy. clin infect dis, 2012. 55(9): p. 1262-9.
[3]. zheng, y., et al., antiretroviral therapy and efficacy after virologic failure on first-line boosted protease inhibitor regimens. clin infect dis, 2014. 59(6): p. 888-96.
[4]. falcoz, c., et al., pharmacokinetics of gw433908, a prodrug of amprenavir, in healthy male volunteers. j clin pharmacol, 2002. 42(8): p. 887-98.
Fosamprenavir calcium Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(131)Suppliers
-
CONIER CHEM AND PHARMA LIMITED
Telefon +8618523575427
E-Mail sales@conier.com
-
Telefon +86-13806087780
E-Mail sale@simagchem.com
-
Telefon +1-781-999-5354<br/>+1-00000000000
E-Mail marketing@targetmol.com
-
Telefon +86-0371-86658258<br/>+8613203830695
E-Mail laboratory@coreychem.com
-
Telefon +86-0571-85134551
E-Mail sales@afinechem.com
-
Dayang Chem (Hangzhou) Co.,Ltd.
Telefon 571-88938639<br/>+8617705817739
E-Mail info@dycnchem.com
-
Zhejiang J&C Biological Technology Co.,Limited
Telefon +1-2135480471<br/>+1-2135480471
E-Mail sales@sarms4muscle.com
-
Telefon +86-0571-81612335<br/>+8613336028439
E-Mail sales@nextpeptide.com
-
Telefon +1-708-310-1919<br/>+1-13798911105
E-Mail sales@invivochem.cn
-
Hefei TNJ Chemical Industry Co.,Ltd.
Telefon +86-0551-65418684<br/>+8618949823763
E-Mail sales@tnjchem.com
1of2
