Synthese
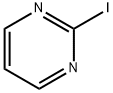
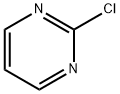
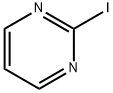
Under nitrogen protection, 2-chloropyrimidine (200 g, 1.75 mol) was slowly added in five portions to an aqueous solution of hydriodic acid (850 ml, 57%) pre-cooled to -10 to -5 °C. The reaction temperature was maintained in the range of -10 to -5°C and the progress of the reaction was monitored by high performance liquid chromatography (HPLC). After completion of the reaction (60 to 120 min), the pH was adjusted to 7.25 ± 0.25 with 30% sodium hydroxide solution and the temperature of the reaction mixture was raised to 18-23°C. Subsequently, sodium sulfite (16 g) was added for decolorization and the pH was adjusted to 3 ± 1. Tert-butyl methyl ether (TBME, 600 ml) was added to the mixture and saturated with sodium chloride (300 g). The organic and aqueous phases were separated and the aqueous phase was extracted with tert-butyl methyl ether (4 x 400 ml). The organic layers were combined and washed sequentially with 1% aqueous sodium sulfite (50 ml) and water (100 ml). The organic layers were concentrated to dryness and co-evaporated with tert-butyl methyl ether (100 ml) at 45 to 50 °C to remove residual water. The final product 2-iodopyrimidine 330 g was obtained in 90% yield and 98% purity as determined by HPLC, which was consistent with the standard.