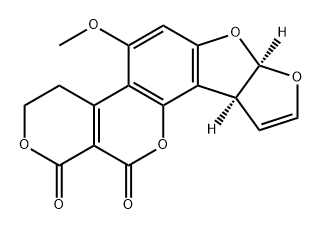
(7aR,cis)3,4,7a,10a-Tetrahydro-5-methoxy-1H,12H-furo(3',2':4,5)-furo(2,3-h)pyrano(3,4-c)(1)-chromen-1,12-dion
Bezeichnung:(7aR,cis)3,4,7a,10a-Tetrahydro-5-methoxy-1H,12H-furo(3',2':4,5)-furo(2,3-h)pyrano(3,4-c)(1)-chromen-1,12-dion
CAS-Nr1165-39-5
Englisch Name:AFLATOXIN G1
CBNumberCB4702106
SummenformelC17H12O7
Molgewicht328.27
MOL-Datei1165-39-5.mol
Synonyma
(7aR,cis)3,4,7a,10a-tetrahydro-5-methoxy-1H,12H-furo[3',2':4,5]furo[2,3-h]pyrano[3,4-c]chromen-1,12-dion
(7aR,cis)3,4,7a,10a-Tetrahydro-5-methoxy-1H,12H-furo(3',2':4,5)-furo(2,3-h)pyrano(3,4-c)(1)-chromen-1,12-dion
(7aR,cis)3,4,7a,10a-Tetrahydro-5-methoxy-1H,12H-furo(3',2':4,5)-furo(2,3-h)pyrano(3,4-c)(1)-chromen-1,12-dion physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 244-246 °C |
| alpha | D -556° (chloroform) |
| Siedepunkt | 386.03°C (rough estimate) |
| Dichte | 1.3358 (rough estimate) |
| Brechungsindex | 1.4790 (estimate) |
| Flammpunkt | -11 °C |
| storage temp. | 2-8°C |
| Löslichkeit | DMF: Soluble; DMSO: Soluble; Ethanol: Soluble; Methanol: Soluble |
| Aggregatzustand | Solid |
| Farbe | White to off-white |
| Biologische Quelle | Aspergillus flavus |
| Wasserlöslichkeit | 15mg/L(temperature not stated) |
| BRN | 1299768 |
| LogP | 0.679 (est) |
| EPA chemische Informationen | Aflatoxin G1 (1165-39-5) |
| Kennzeichnung gefährlicher | T+,T,F,Xn |
| R-Sätze: | 45-26/27/28-65-48/23/24/25-36/38-11-46-39/23/24/25-23/24/25-36-20/21/22 |
| S-Sätze: | 53-28-36/37-45-62-26-16-7-36 |
| RIDADR | UN 3462 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS-Nr. | LV1720000 |
| F | 10 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| HS Code | 29322090 |
| Giftige Stoffe Daten | 1165-39-5(Hazardous Substances Data) |
| Toxizität | LD50 orally in day old duckling: 39.2 mg/50 gm body wt (Carnaghan) |



