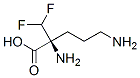
Eflornithine
Bezeichnung:Eflornithine
CAS-Nr67037-37-0
Englisch Name:Eflornithine
CBNumberCB4373229
SummenformelC6H12F2N2O2
Molgewicht182.17
MOL-Datei67037-37-0.mol
Eflornithine Chemische Eigenschaften,Einsatz,Produktion Methoden
-
Beschreibung
Metcalf et al.reported the synthesis of eflornithine (difluoromethyl ornithine [DFMO]) in 1978. Their interest arose from the desire to prepare ornithine decarboxylase (ODC) inhibitors as tools for studying the role of polyamines as regulators of growth processes. Ornithine decarboxylase catalyzes the conversion of ornithine to putrescine (1,4-diaminobutane), which in turn leads to the formation of the polyamines, spermine, and spermidine. It was not until 1980 that Bacchi et al. demonstrated the potential of DFMO in the treatment of trypanosomiasis. -
Verwenden
Eflornithine is an irreversible inhibitor of ornithine decarboxylase that blocks putrescine biosynthesis in T. brucei in vitro and inhibits the growth and multiplication of the parasite in vivo. The drug, administered orally or parenterally, is relatively nontoxic and is effective in eliminating the parasite T. b. rhodesiense and, to a greater extent, T. b. gambiense. Eflornithine was approved for human use by the FDA in late 1990. Side effects associated with oral administration are diarrhea, nausea, and vomiting. There is a significant relapse rate. A mixture of eflornithine (5) and nifurtimox (6, C10H13N3O5S, Lampit, Bayer 2502) shows good activity against T. b. gambiense. The former compound is also reported to potentiate the effect of melarsoprol in infected mice. -
Verwenden
Labelled Eflornithine. Irreversible inhibitor of ornithine decarboxylase, an enzyme involved in polyamine biosynthesis. Antineoplastic; antipneumocystic; antiprotozoal (Trypanosoma). Used in the treatment of hirsutism. -
Indications
Eflornithine (difluoromethyl ornithine, Ornidyl) is a unique antiprotozoal agent in that its mode of action involves inhibition of a specific enzyme, ornithine decarboxylase. In eukaryotes, decarboxylation of ornithine is required for biosynthesis of polyamines, which are important in cell division and differentiation.
Eflornithine is given intravenously, and about 80% of the drug is excreted in the urine within 24 hours. It does not bind significantly to plasma proteins and has a terminal plasma half-life of about 3 hours. It crosses the blood-brain barrier and is one of the drugs of choice for treating the hemolymphatic and meningoencephalitic stage of T. brucei-gambiense. The most significant side effects are anemia and leukopenia. Oral therapy is associated with considerable gastrointestinal toxicity. Diarrhea, thrombocytopenia, and seizures are occasionally reported. -
Definition
ChEBI: Eflornithine is a fluoroamino acid that is ornithine substituted by a difluoromethyl group at position 2. It has a role as a trypanocidal drug. It is a fluoroamino acid and an alpha-amino acid. It is functionally related to an ornithine. -
Trademarks
Ornidyl (Sanofi Aventis); Vaniqa (Skinmedica). -
Mechanism of action
Difluoromethyl ornithine is a suicide inhibitor of ODC, a pyridoxal phosphate–dependent enzyme. Evidence suggests that cysteine-360 in ODC is the site of eflornithine alkylation. Alkylation of ODC blocks the synthesis of putrescine, the rate-determining step in the synthesis of polyamines. Mammalian ODC also may be inhibited, but because the turnover of ODC is so rapid in mammals, eflornithine does not produce serious side effects. -
Clinical Use
Eflornithine is used for the treatment of West African sleepingsickness, caused by Trypanosoma brucei gambiense.It is specifically indicated for the meningoencephaliticstage of the disease. Eflornithine is a myelosuppressivedrug that causes high incidences of anemia, leukopenia,and thrombocytopenia. Complete blood cell counts must bemonitored during the course of therapy.The irreversible inactivation of ornithine decarboxylaseby eflornithine is accompanied by decarboxylation andrelease of fluoride ion from the inhibitor, suggesting enzyme-catalyzed activation of the inhibitor. Only the (—) isomer,stereochemically related to L-ornithine, is active.
Eflornithine is supplied as the hydrochloride salt. It may beadministered either intravenously or orally. Approximately80% of the unchanged drug is excreted in the urine.Penetration of eflornithine into the CSF is facilitated by inflammationof the meninges. -
Nebenwirkungen
Side effects reported for eflornithine consist of anemia, diarrhea, and leukopenia.
Eflornithine Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(41)Suppliers
-
Hubei xin bonus chemical co. LTD
Telefon 86-13657291602
E-Mail linda@hubeijusheng.com
-
CONIER CHEM AND PHARMA LIMITED
Telefon +8618523575427
E-Mail sales@conier.com
-
Shaanxi Dideu Medichem Co. Ltd
Telefon +86-029-89586680<br/>+86-18192503167
E-Mail 1026@dideu.com
-
Dayang Chem (Hangzhou) Co.,Ltd.
Telefon 571-88938639<br/>+8617705817739
E-Mail info@dycnchem.com
-
Hefei TNJ Chemical Industry Co.,Ltd.
Telefon +86-0551-65418684<br/>+8618949823763
E-Mail sales@tnjchem.com
-
Telefon +8618058761490
E-Mail info@gihichemicals.com
-
XIAMEN AMITY INDUSTRY AND TRADE CO., LTD.
Telefon +8618950047208
E-Mail ellena@amitychem.com
-
Pure Chemistry Scientific Inc.
Telefon 001-857-928-2050 or 1-888-588-9418
E-Mail sales@chemreagents.com
-
Chemsky (shanghai) International Co.,Ltd
Telefon 021-50135380
E-Mail shchemsky@sina.com
-
Hubei Jusheng Technology Co.,Ltd
Telefon 027-59599241<br/>18871490274
E-Mail 1400878000@qq.com
1of2
