Manufacturing Process
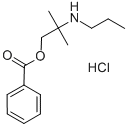
78 g of N-(1,1-dimethyl-2-hydroxy-ethyl)propylamine is added to a solution of 30 g of sodium hydroxide in 700 ml of water. To this is added 300 ml of ether and this is followed by the dropwise addition of 70 ml of benzoyl chloride benzoyl chloride while stirring and cooling. The benzoyl chloride is added at such a rate that the temperature does no rise above 30°C. When the addition is complete, the stirring is continued for another 30 minutes. The aqueous layer is removed and the ether layer is washed with water, dried and evaporated to leave an yellow oil 1-propanol, 2-methyl-2-(propylamino)-, benzoate. This is treated slowly with 45 ml of concentrated hydrochloric acid, during which addition a vigorous exothermic reaction ensues. When the reaction mixture is cooled, it solidifies to a pasty solid which is allowed to dry. This is dissolved in boiling isopropanol and allowed to cool when white crystals of the hydrochloride are formed. The resultant slurry is filtered and the hydrochloride of 1-propanol, 2-methyl-2-(propylamino)-, benzoate recrystallized from isopropanol to give white crystals; MP: 150°-151°C. It is useful in base and salt forms as such as a topical local anesthetic being administrable in oil or alcohol solution.
In practice it is usually used as hydrochloride.
Safety Profile
Poison by subcutaneous, intravenous, and intraperitoneal routes. When heated to decomposition it emits toxic fumes of NOx and HCl