The general procedure for the synthesis of 1aH-dibenzo[b,f]oxovinyl[2,3-d]azepine-6(10bH)-carboxamide from 5H-dibenzo[b,f]azepine-5-carboxamide is as follows:
Example 1: Synthesis of IIa,10b-dihydro-6H-dibenzo[b,f]oxoethene[d]azepine-6-carboxamide (5); potassium permanganate loaded on alumina was added to a stirred suspension of carbamazepine (3) (200 g, 847.5 mmol) and sodium carbonate (287.4 g, 271 mmol) in dichloromethane (1000 ml) tablets (3.5% w/w, 3.46 g, 0.77 mmol). Subsequently, peroxyacetic acid (39% solution in acetic acid, 432 ml, 2538 mmol) was added dropwise over a period of 1 h. The temperature was controlled to gradually increase until the solvent was mildly refluxed. The reaction mixture was stirred for 20 min and then left to stand for 20 min. The sodium carbonate and loaded catalyst were removed by filtration and washed with dichloromethane (200 ml); the alumina beads were separated from the sodium carbonate by sieving. The combined filtrates were stirred with a solution of sodium sulfite (20 g) and sodium bicarbonate (20 g) in water (250 ml) for 1 hour. The organic and aqueous phases were separated and the aqueous phase was extracted with dichloromethane (50 ml). The combined organic layers were washed sequentially with water (100 ml), saturated aqueous sodium bicarbonate solution (100 ml), water (100 ml) and brine, then dried with anhydrous sodium sulfate and filtered. The solvent was evaporated in a rotary evaporator (absorber pressure, 40°C) to give the crude epoxide (5) as a beige solid. The crude product was recrystallized by ethyl acetate (100 ml) to give an off-white solid product with a yield of 194.2 g in 91% yield.
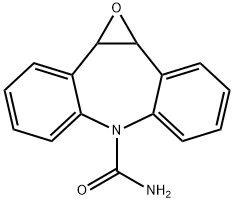
![1A,10B-DIHYDRO-6H-DIBENZO[B,F]OXIRENO[D]AZEPINE-6-CARBOXAMIDE](/CAS/GIF/36507-30-9.gif)