Description
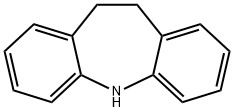
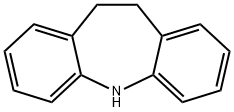
Iminodibenzyl was not a new drug. It had been discovered in 1898 and used briefly as an intermediate, in the preparation of Sky Blue, a dye stuff. Iminodibenzyl, however, had a tricyclic ring structure, similar in appearance to the phenothiazines. Iminodibenzyl is an important drug intermediate, which can be used for the medicine synthesis as a tristimania and epilepsia.
Chemical Properties
YELLOWISH TO BEIGE CRYSTALLINE POWDER
Uses
10,11-Dihydro-5H-dibenzo[b,f]azepine is a metabolite of the tricyclic antidepressant, Imipramine (I465980). 10,11-Dihydro-5H-dibenzo[b,f]azepine can be used as a chromogenic probe for the quantification of hydrogen peroxide and glucose.
Preparation
Iminodibenzyl is obtained from o-nitrotoluene by condensation, reduction and cyclization.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Synthesis
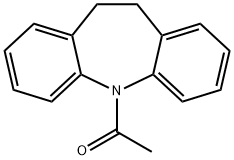
The general procedure for the synthesis of iminodibenzylidene from 1-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)ethanone is as follows: NaOH and triethylboron were stirred and mixed under argon protection at room temperature to form a clear, clarified solution with a concentration of 1 M/L. Subsequently, 10 μmol (2 mol%) of catalyst, 5 mmol of amide substrate, and 15 mmol of triethoxysilane or polymethylhydrosiloxane (PMHS) were sequentially added to the above triethylboron solution. The reaction mixture was added to a 10 mL sealed tube along with 2 mL of methyl tert-butyl ether (MTBE) and placed in an 80 °C oil bath with heating and stirring for 6 hours. Upon completion of the reaction, the reaction system was exposed to air and the amine products were subsequently isolated directly by column chromatography. According to the column chromatographic separation, the yield of iminodibenzyl was when triethoxysilane or polymethylhydrosiloxane (PMHS) was used as the reducing agent:
References
[1] Patent: CN107337573, 2017, A. Location in patent: Paragraph 0022; 0023; 0024; 0025