Originator
Fenproporex,Chephasaar,W. Germany,1975
Manufacturing Process
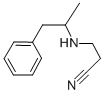
(a) 22 g of acrylonitrile and 27 g of racemic α-methyl-β-phenylethylamine
were introduced into a 100 ml round-bottomed flask and left standing for 13
hours at ambient temperature, and then the mixture was boiled under reflux
for 12? hours. The excess acrylonitrile was then evaporated in vacuo and the
residue distilled. 27.3 g (yield: 72.6%) of racemic N-(β-cyanoethyl)-α-methyl-
β-phenylethylamine were obtained as an oily liquid, BP = 126°C to 127°C/2
mm Hg.
(b) 22 g of the base obtained in (a) were dissolved in 80 ml of anhydrous
diethyl ether and an ethereal solution of hydrochloric acid added until the pH
value was 1. The salt was filtered off, dried and washed with 10 ml of diethyl
ether. 18 g (yield: 68%) of N-(β-cyanoethyl-α-methyl-β-phenylethylamine
hydrochloride were obtained, after recrystallization from absolute ethanol, as
a white, microcrystalline, odorless powder having a bitter, acid taste; it was
fairly soluble in water, ether and benzene. MP = 146°C on a Kofler block.