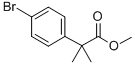
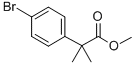
Sodium hydride (60% oil dispersion, 192 mg, 4.80 mmol) was added to dry DMF (11 mL) at 0 °C to form a suspension. Subsequently, methyl 2-(4-bromophenyl)acetate (0.35 mL, 2.2 mmol) was slowly added to this suspension. After the reaction mixture was stirred continuously for 15 min at 0 °C, iodomethane (6.5 μL, 0.10 mmol) was added dropwise. After addition, stirring was continued at 0 °C for 5 min, and then raised to room temperature and stirred for 12 hours. After completion of the reaction, the reaction was quenched with 1N HCl aqueous solution (1 mL) and extracted with ethyl acetate (3 × 2 mL). The organic layers were combined, washed sequentially with water (3 × 2 mL) and saturated saline (2 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Finally, the residue was purified by silica gel column chromatography (eluent: 0% to 5% ethyl acetate/hexane gradient elution) to afford the target product methyl 2-(4-bromophenyl)-2-methylpropionate (550 mg, 98% yield); mass spectrometry (electrospray positive ionization mode) C10H11BrO2 Calculated value: 242/244, measured value: 243/245 [M+H]+.