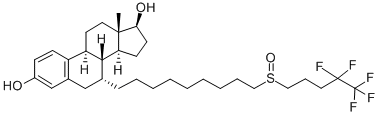
Description
Fulvestrant was launched in the US as a novel once monthly injectable steroidal
estrogen antagonist for the treatment of hormone receptor positive metastatic breast
cancer in postmenopausal women with disease progression following estrogen therapy.
This 7a-alkylsulphinyl derivative of estradiol can be prepared in 10 steps from 6,7-
didehydro-19-nor-testosterone by successive conjugate addition of the organocuprate
derived from O-protected 9-bromononan-l-o1 followed by aromatization of the resulting
enone, then activation of the protected primary alcohol, substitution with 4,4,5,5,5-
pentafluoropentanthiol and oxidation to the sulfoxide. Fulvestrant is the first “pure”
estrogen antagonist from a novel class known as selective estrogen receptor down
regulators (SERDs). It binds to the estrogen receptor (ER), with affinity close to that of
estradiol and 100 fold greater than that of tamoxifen (a partial estrogen antagonist),
preventing estrogen-stimulated gene activation, thereby interfering with the estrogenrelated
processes essential for cell-cycle completion. Fulvestrant also appears to
downregulate the ER by 80-90% often to non detectable level both in vitro and in vivo. In
comparison to tamoxifen, fulvestrant is devoid of systemic estrogenic activity, it displays no
uterotrophic activity and is able to block the uterine stimulation of estradiol or tamoxifen.
Furthermore, fulvestrant completely blocks the cell growth in tamoxifen-resistant breast
cancer cell-lines and prevents growth of tamoxifen resistant tumor in mice. In clinical trials,
it was also shown that fulvestrant is comparable to anastrozole (a third generation
aromatase inhibitor) both in efficacy and tolerability in postmenopausal women with
tamoxifen-resistant advanced breast cancers.
Chemical Properties
White or almost white powder.
Originator
Astra Zeneca (UK)
Uses
A novel steroidal estrogen antagonist reported to lack any partial agonist activity. Antineoplastic (hormonal).
Definition
ChEBI: A 3-hydroxy steroid that is 17beta-estradiol in which the 7alpha hydrogen has been replaced by a nonyl group in which one of the hydrogens of the terminal methyl has been replaced by a (4,4,5,5,5-pentafluoropentyl)sulfinyl
group. An estrogen receptor antagonist, it is used in the treatment of breast cancer.
Brand name
Faslodex (AstraZeneca).
General Description
Fulvestrant, 7α-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol (Faslodex), is an antagonist structurally based onthe estradiol structure, with a long, substituted alkyl chainattached at the 7α-position of the steroid skeleton. Whenbound to the ERs, this alkyl chain induces a conformationof the receptor distinctive from that formed upon estradiolor tamoxifen binding, preventing agonist action.Fulvestrant is a pure antagonist at both ERαand ERβandan ER downregulator (stimulates degradation of the ER),completely lacking the agonist activity that is seen with tamoxifenor raloxifene. The different pharmacological profileof fulvestrant allows the use of this agent in womenwho have had disease progression after prior antiestrogentherapy (typically tamoxifen), providing an alternative toaromatase inhibitors.
Biological Activity
A high affinity estrogen receptor antagonist (IC 50 = 0.29 nM), devoid of any partial agonism both in vitro and in vivo . Also high affinity agonist at the membrane estrogen receptor GPR30.
Biochem/physiol Actions
Fulvestrant (ICI 182,780) is a selective estrogen receptor down-regulator (SERD). Fulvestrant is a high affinity estrogen receptor antagonist. IC50 = 0.29 nM. Fulvestrant is the first "pure" antiestrogen with no agonistic activity both in vitro and in vivo.
Clinical Use
Treatment of postmenopausal women with oestrogenreceptor-
positive, locally advanced or metastatic breast
cancer
Side effects
Side effects appear to be minimal and include several GI symptoms , headache, and hot flashes . There is no clinical evidence of uterine stimulation or laboratory evidence of stimulation of endometrial carcinoma models. Fulvestrant should not be adm inistered to women who are pregnant, who are taking antic oagulants, or who have thrombocytopenia.
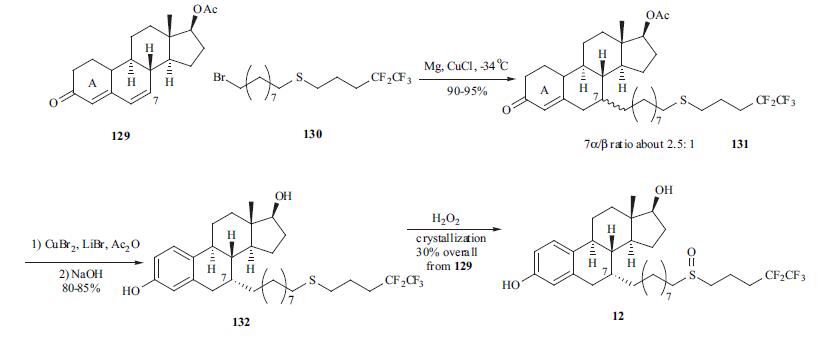
Synthesis
Fulvestrant is administered as a once a month
i. m. injection. Several routes for the synthesis of fulvestrant
(12) were published. One of the best routes is
depicted in the scheme. The conjugate addition of Grignard
reagent derived from bromide 130 with dienone 129 gave
adduct 131 as a mixture of 7|á- and 7|?-isomers in a ratio of
2.5:1 in 90-95% yield. Aromatization of the A-ring with
copper bromide/lithium bromide in acetic acid followed by
hydrolysis of the ester group provided diol 132 in 80-85%
yield. Oxidation of the side chain from sulfite to sulfone
followed by crystallization provided fulvestrant (12) in 30%
overall yield from dienone 129.
Drug interactions
Potentially hazardous interactions with other drugs
None known
Metabolism
The metabolism of fulvestrant has not been fully
evaluated, but involves combinations of a number of
possible biotransformation pathways analogous to those
of endogenous steroids. Identified metabolites (includes
17-ketone, sulphone, 3-sulphate, 3- and 17-glucuronide
metabolites) are either less active or exhibit similar
activity to fulvestrant in anti-oestrogen models.
Fulvestrant is eliminated mainly in metabolised form. The
major route of excretion is via the faeces.
References
Osborne et al. (2004), Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action; Br. J. Cancer 90 (Suppl 1):S2
Thomas et al. (2005), Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells; Endocrinology 146 624
Wardley (2002), Fulvestrant: a review of its development, pre-clinical and clinical data; Int. J. Clin. Pract. ?56 305
Castro et al. (2012),?Coumestrol has neuroprotective effects before and after cerebral ischemia in female rats; Brain Res.?1474 82
Blackburn et al. (2018),?Fulvestrant for the treatment of advanced breast cancer; Expert Rev. Anticancer Ther. 18 619