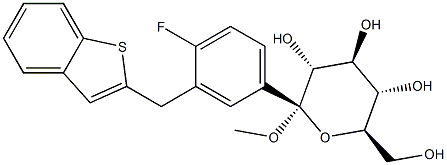
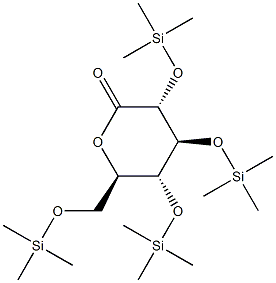
A tetrahydrofuran solution of 0.9 M n-butylmagnesium chloride (0.9 M, 7.3 mL) was slowly added to toluene (18.0 mL) at -16 °C. Subsequently, n-hexane solution of n-butyllithium (2.66 M, 4.9 mL) was added dropwise at -12 °C and stirred for 16 min in the range of -12 to -15 °C. 2-(5-bromo-2-fluorobenzyl)benzo[b]thiophene (Compound I, 2.000 g) was dissolved in toluene (10.0 mL) and added dropwise to the reaction system at -12 to -15 °C, the dropwise process lasted for 1 hour. Next, a toluene solution (10.0 mL) of (3S,4S,5R,6R)-3,4,5-tris((trimethylmethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one (Compound II, 3.197 g) was added dropwise to the reaction solution and stirred for 3 hours at -12 to -15 °C. Upon completion of the reaction, the mixture was poured into a methanol solution (10.0 mL) of methanesulfonic acid (3.0 mL) at 0 °C or lower, followed by stirring for 16 hours and 36 minutes at room temperature. The reaction mixture was quenched with an aqueous sodium carbonate solution of pH about 8. The organic layer was washed with saturated brine (10 mL, once), dried over anhydrous sodium sulfate, and extracted with ethyl acetate (50 mL, twice). After filtration, the solvent was removed by distillation under reduced pressure and the resulting residue was purified by silica gel column chromatography to afford the target product (2S,3R,4S,5S,6R)-2-(3-(benzo[b]thiophene-2-ylmethyl)-4-fluorophenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5triol (2.336 g, 86.4% yield).

![Benzo[b]thiophene, 2-[(5-broMo-2-fluorophenyl)Methyl]-](/CAS/20150408/GIF/1034305-17-3.gif)
![Methyl 1-C-[3-(benzo[b]thien-2-ylMethyl)-4-fluorophenyl]-](/CAS/20150408/GIF/1034305-23-1.gif)