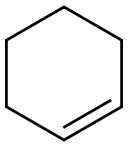
What is Cyclohexene?
Cyclohexene (C6H10, CAS registry No. 110-83-8) is also called tetrahydrobenzene, which is colorless flammable liquid. It is insoluble in water. Inhalation of high concentrations may have a narcotic effect. Cyclohexene is highly flammable, so it should keep away from heat and open flame. It may form peroxides in storage, so it should be stored in the absence of air. It may cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases.
Cyclohexene may react vigorously with strong oxidizing agents. Though simple in its chemical structure, there are two potential oxidation sites in cyclohexene, and the usual oxidation reactions generally lead to a mixture of products with different oxidation states and functional groups: oxidation of the C=C bond (site a) can lead to 7-oxabicyclo[4.1.0]heptane, trans/cis-cyclohexane-1,2-diol, or adipic acid; oxidation at the allylic C-H position (site b) may produce cyclohex-2-en-1-ol or cyclohex-2-en-1-one. These products are useful industrial intermediates that have been widely employed in organic synthesis, medicinal chemistry, pesticide chemistry, materials science, etc. Therefore, controllable oxidation reactions for cyclohexene that can selectively afford the targeted products are synthetically valuable for applications in both the academy and industry, thus becoming the aim of synthetic and catalytic chemists in the field. Many reports on selective oxidation of cyclohexene have recently appeared in the literature because of its significance[1].
Cyclohexene may react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions.
Cyclohexene with an active double bond is a cheap, abundant, and easily accessible raw material in industry, which is widely used in the synthesis of pharmaceuticals, pesticides, food and other high value-added fine chemicals. Cyclohexene can be synthesized by several ways, such as dehydrogenation of cyclohexane, dehydration of cyclohexanol, dehydrohalogenation of halogenated cyclohexane, Birch reduction or partial hydrogenation of benzene. Compared with other methods,the technology of partial hydrogenation of benzene to cyclohexene is of the advantage of safety, high atom-economy, environmentally friendly[2]. The partial hydrogenation of benzene to cyclohexene has been known for more than 100 years. In 1957, it was identified in the products of the hydrogenation of benzene catalyzed by Ni membrane. Since then, the production of cyclohexene in the hydrogenation of benzene at low degrees of conversion has been reported by several researchers, and ruthenium catalysts have been especially suiting for the reaction. One of the most important methods was the production of cyclohexene in the tetra-phase reactors containing an organic phase, an aqueous phase, a gas phase (H2) and a solid phase (catalysts), catalyzed by transition metal salts. And the yields of cyclohexene can achieve as high as 60%. In 1989, the Ru-Zn catalyst for benzene partial hydrogenation was industrialized for the first time in Japan by Asahi-Kasei Chemical Co., Ltd. The technology was introduced from Japan into Shenma Group Company in 1995. In recent years, a great number of scientific articles show that the selectivity of cyclohexene could be improved by reasonably designing ruthenium catalysts. For example, a series of Ru-Zn/ZrO2 catalysts are prepared by post-treatment of a binary Ru-catalyst using NaOH aqueous solutions. Alkaline post-treatment removed metallic Zn, the surface hydroxyl groups content and the hydrophilicity of the catalysts were increased.
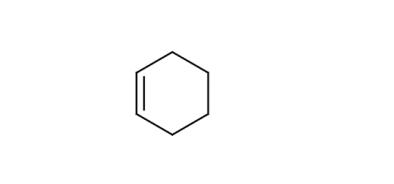
Fig 1. Chemical structure formula of cyclohexene
References
[1]. Cao, H.; Zhu, B.; Yang, Y.; Xu, L.; Yu, L.; Xu, Q., Recent advances on controllable and selective catalytic oxidation of cyclohexene. Chinese Journal of Catalysis 2018, 39 (5), 899-907.
[2]. Hongqin, W.; jiyang, X.; Nihong, A.; yunsheng, D.; Chun, T.; Jun, L.; Yafeng, S.; Wei, Z., Advances in the Ruthenium Catalysts for Partial Hydrogenation of Benzene to Cyclohexene. Meterials Reports 2019, 33 (12), 4016-4024.
);You may like
Lastest Price from Cyclohexene manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 110-83-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00/KG2023-08-16
- CAS:
- 110-83-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month