Synthesis and Preparation of Muscone
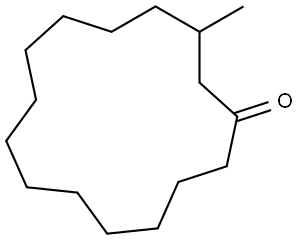
(R)-(-)-Muscone (3-methylcyclopentadecan-1-one) is the main odorous principle of musk pod obtained from the male musk deer[2] for perfumes and colognes. (R)-Muscone has a very nice musky, rich powerful fragrance, Due to overhunting, the musk deer is now a protected species, therefore a conventional chemical synthesis of homochiral (R)-muscone is needed[3].
The key synthetic challenges of (R)-muscone are the construction of the macrocyclic ring and the establishment of the chiral methyl group. In racemic form muscone has been synthesized by many routes, which mainly dealt with the construction of the 15-membered ring, involving a variety of strategies such as ring expansion methods, acyloin condensation and fragmentation reactions[4].
Its absolute configuration was established by an asymmetric synthesis via the electrolytic method using monomethyl ester of tridecanedioic acid and b-methylglutaric acid and further confirmed by ORD studies[2].
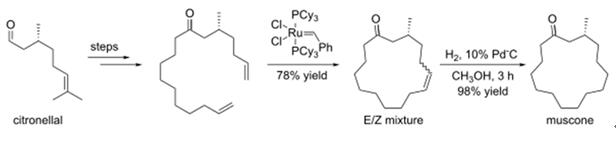
One asymmetric synthesis of (R)-(-)-muscone begins with commercially available (+)-citronellal, and forms the 15-membered ring via ring-closing metathesis[2]:
And the idea is feasible and applicable to the synthesis of enantiomerically pure (R)-muscone (8, Scheme 11) by an asymmetrically catalyzed macrocyclization of an ω-alkynal[5]:
A study about an enantioselective synthesis of (R)-muscone involved an intramolecular aldol addition/dehydration reaction of a macrocyclic diketone is developed[6].
Many interesting ways to introduce the chiral methyl group of (R)-Muscone are also studied.
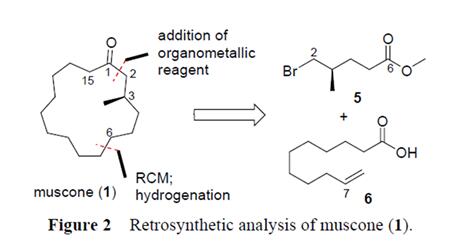
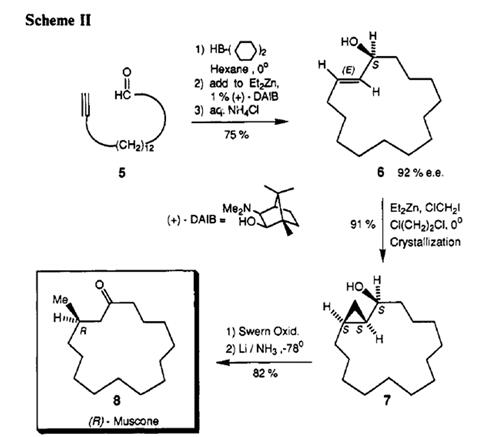
The stereogenic center of muscone was introduced from methyl (R)-5-bromo-4-methylpentanoate (5), a chiral pool molecule, and the macrocyclic ring was prepared by ring-closing metathesis (RCM) reaction. A retrosynthetic analysis of muscone (1) is outlined in Figure 2. Macrocycle of muscone was to be closed by the RCM reaction at C(6)-C(7) position, and its precursor by a nucleophilic addition of organometallic reagent derived from 5 to undec-10-enoic acid 6,
which is very cheap and available in large quantity[4].
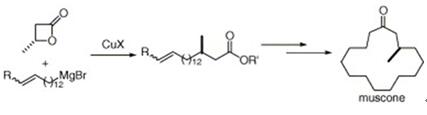
Chiral (R)-muscone was also constructed via ring-opening reaction of (R)-(+)-β-methyl-β-propiolactone with functionalized organocuprate with almost no loss of optical purity[3].
References
[1]http://www.chemspider.com/Chemical-Structure.5499607.html
[2]Vijayendra P. Kamat, Hisahiro Hagiwara, Tomoko Katsumi, Takashi Hoshi, Toshio Suzuki, Masayoshi Ando. Ring Closing Metathesis Directed Synthesis of (R)-(-)-Muscone from (+)-Citronellal[J]. tetrahedron, 2000, 56: 4397-4403.
[3]Motoaki Morita, Nobuyuki Mase, Hidemi Yoda, Kunihiko Takabe. A simplified synthesis of (R)-(-)-muscone using a ring-opening reaction of (R)-(+)-b-methyl-b-propiolactone[J]. Tetrahedron: Asymmetry, 2005, 16: 3176-3182.
[4]Junwei Shen, Yong Shi, Weisheng Tian. Synthesis of (R)-(−)-Muscone from (R)-5-Bromo-4-methylpentanoate: A Chiron Approach[J]. Chin. J. Chem. 2015, 33: 683-687.
[5]Wolfgang Oppolzer, Rumen N. Radinov. Synthesis of (R)-(-)-Muscone by an Asymmetrically Catalyzed Macrocyclization of an ω-alkynal [J]. J. Am. Chem. SOC, 1993, 115: 1593-1594.
[6]Oliver Knopff, Jerome Kuhne, Charles Fehr. Enantioselective Intramolecular Aldol Addition/Dehydration Reaction of a Macrocyclic Diketone: Synthesis of the Musk Odorants (R)-Muscone and (R,Z)-5-Muscenone[J]. Angew. Chem. Int. Ed. 2007, 46: 1307-1310.
You may like
Related articles And Qustion
See also
Lastest Price from Muscone manufacturers

US $50.00/kg2024-04-23
- CAS:
- 541-91-3
- Min. Order:
- 1kg
- Purity:
- 99.10%
- Supply Ability:
- 50000kg

US $100.00/drum2023-09-14
- CAS:
- 541-91-3
- Min. Order:
- 1drum
- Purity:
- 99
- Supply Ability:
- 5000