Sugammadex: A revolutionary drug in neuromuscular pharmacology
Sugammadex (ORG 25969) is a unique neuromuscular reversal drug; a novel cyclodextrin, the first in a new class of selective relaxant binding agents, which reverse neuromuscular blockade (NMB) with the aminosteroid non-depolarizing muscle relaxants rocuronium and vecuronium. Sugammadex can reverse moderate or deep NMB. The clinical use of sugammadex promises to eliminate many of the shortcomings in current anesthetic practice with regard to antagonism of rocuronium and other aminosteroid muscle relaxants.
CHEMICAL STRUCTURE
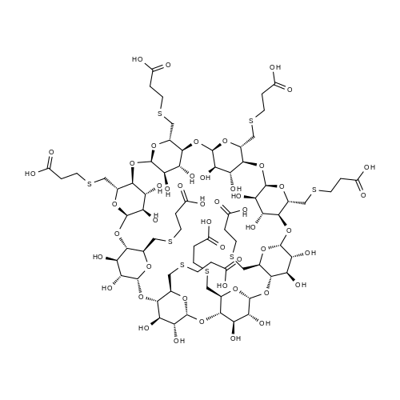
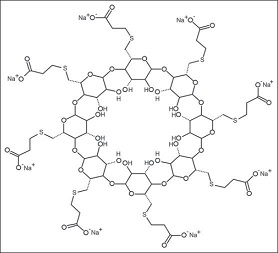
Sugammadex [6A,6B,6C,6D,6E,6F,6G,6H-octakis-S-(2-carboxyethyl)-6A,6B,6C,6D,6E,6F,6G,6H-octathio-γ-cyclodextrin octasodium salt] is a modified γ-cyclodextrin [Figure 1]. Chemical formula is C72H104Na8O48S8. “Su” stands for sugar and “gammadex” stands for structural molecule gamma-cyclodextrin.[1] Cyclodextrins are cyclic dextrose units joined through 1-4 glycosyl bonds that are produced from starch or starch derivates using cyclodextrin glycosyltransferase. The three natural unmodified cyclodextrins are α-, β-or γ-cyclodextrin. Compared with α-and β-cyclodextrins, γ-cyclodextrin exhibits more favorable properties in terms of the size of its internal cavity, water solubility and bioavailability. To have a better fit of the larger rigid structure of the aminosteroid muscle relaxant molecule (e.g., rocuronium or vecuronium) within the cavity of γ-cyclodextrin, the latter was modified by adding eight side chains to extend the cavity. This modification allowed the four hydrophobic steroidal rings of rocuronium to be better accommodated within the hydrophobic cavity. In addition, adding negatively charged carboxyl groups at the end of the eight side chains served two purposes. First, the repellent forces of the negative charges keep propionic acid side chains from being disordered, thereby allowing the cavity to remain open and maintain structural integrity. Second, adding these negatively charged carboxyl groups enhances electrostatic binding to the positively charged quaternary nitrogen of rocuronium [Figure 1]. Three-dimensional structure resembles a hollow, truncated cone or a doughnut.[9]
Mechanism of action
Inactivates rocuronium by encapsulating (chelating) the free molecule to form a stable complex.[10] The structure has a hydrophobic cavity and a hydrophilic exterior because of the presence of polar hydroxyl groups. Hydrophobic interactions trap the drug into the cyclodextrin cavity, resulting in the formation of a water-soluble guest–host complex. Sugammadex exerts its effect by forming very tight complexes at a 1:1 ratio with aminosteroid muscle relaxants (rocuronium > vecuronium >> pancuronium).[9,10,11,12] The intermolecular (van der Waals’) forces, thermodynamic (hydrogen) bonds and hydrophobic interactions make the sugammadex–rocuronium complex very tight. The sugammadex–rocuronium complex has a very high association rate (an association constant of 107 M-1) and a very low dissociation rate. It is estimated that, for every 25 million sugammadex–rocuronium complexes, only one complex dissociates. The resulting reduction in free rocuronium plasma concentration creates a gradient between the tissue compartment (including the NMJ) and plasma; free rocuronium moves from tissue to plasma, with a reduction in nicotinic receptor occupancy at the NMJ.[10,13,14]
Metabolism and elimination
The drug does not produce any metabolites and is mostly excreted through urine in the unchanged form within 24 h. An insignificant part is excreted through feces or in expired air (0.02%). The plasma clearance of the drug ranges from 84 to 138 ml/min.[2]
The drug is available in IV formulation only; vials of 200 mg (2 ml) or 500 mg (5 ml) and is for non-refrigerated storage.[19] The drug was originally designated as Org 25,969. Trade name is Bridion. Sugammadex was discovered at the Newhouse research site in Scotland. Scientists who discovered Sugammadex worked for the pharmaceutical company, Organon. Organon was acquired by Schering-Plough in 2007; Schering-Plough merged with Merck in 2009. Sugammadex is now owned and sold by Merck. On January 3, 2008, Schering-Plough submitted a New Drug Application to the US FDA for sugammadex, but was rejected on August 2008.[20,21] It was approved for use in the European Union on July 29, 2008.[17] The drug is also approved for use in Australia, Iceland, New Zealand and Norway.
Indication
Reversal of neuromuscular blockade (NMB) in the operation theater and in the intensive care unit (ICU).[17]
Drug interactions Toremifene, a chemotherapeutic drug, which is a selective estrogen receptor modulator, can delay the reversal by displacing the formation of the rocuronium-sugammadex complex. The drug can theoretically bind with contraceptive steroids. As endogenous steroids and steroidal drugs lack the quaternary nitrogen of the aminosteroid muscle relaxants, they show a much lower affinity. Rocuronium-sugammadex complex can also be displaced by fusidic acid or high doses of flucloxacillin. Drugs like magnesium, aminoglycosides potentiate muscle relaxants; to counteract these effects, a larger dose of sugammadex may be required. Among anesthetic drugs the highest affinity constant was observed with remifentanil (0.2% of the affinity constant of sugammadex with rocuronium); it has not been shown to be clinically significant. Physical incompatibility has been reported with verapamil, ondansetron and ranitidine. Further investigations are required to assess other possible drug interactions.[1,2,9]
Sugammadex does not alter laboratory tests in general, but some modification of the serum progesterone assay and of the coagulation parameters like activated partial thromboplastin time, prothrombin time, INR has been noticed.[17]
Contraindications
Hypersensitivity to sugammadex or to any excipients.[13]
Special considerations Patients with severe renal impairment: Clearance of the drug is reduced in patients with creatinine clearance below 30 ml/min. The efficacy of the drug is not reduced in these patients.[22] There are conflicting reports of its safety in these subset of patients; hence, it is recommended to avoid its use in such patients.[2]
Patients with hepatic impairment: Sugammadex-rocuronium complex is not eliminated by hepatic metabolism; therefore, hepatic impairment does not affect its excretion. However, it is advised to be cautious regarding its use in these patients.[2]
Obese patients: Use adult dose based on actual body weight.
Patients in ICU: Sugammadex has not been investigated in patients receiving rocuronium or vecuronium in the ICU.
Elderly: Use adult dose although recovery times are slower.
Pediatric patients: Pharmacokinetic profile is quite similar to that of adults. There is no clinical trial in children below 2 years of age.
Pregnancy and lactation: Caution in pregnant women. Sugammadex can be used during lactation.
Sex, race and body weight: They have no effect on the use of sugammadex.
Adverse effects
Sugammadex is a well-tolerated and relatively free of adverse effects. Some of the adverse reported in some of the trials are dysgeusia (metal or bitter taste) mainly seen after doses of 32 mg/kg or higher, recurrence of block, movement of limbs or body or coughing during anesthesia, coughing, grimacing or suckling on the endotracheal tube, procedural hypotension.[17,27,28]
Precautions
If re-administration of rocuronium or vecuronium is required a waiting time of 24 h is recommended. If NMB is required before the recommended waiting time has passed, a non-steroidal muscle relaxant should be used. Sugammadex should not be used to reverse block induced by non-steroidal blockers such as suxamethonium or benzylisoquinolinium compounds (because it cannot form inclusion complexes with these drugs) or aminosteroid muscle relaxants other than rocuronium or vecuronium.[9,16]
REFERENCES
1. Kovac AL. Sugammadex: The first selective binding reversal agent for neuromuscular block. J Clin Anesth. 2009;21:444–53. [PubMed] [Google Scholar]
2. Hemmerling TM, Zaouter C, Geldner G, Nauheimer D. Sugammadex: A short review and clinical recommendations for the cardiac anesthesiologist. Ann Card Anaesth. 2010;13:206–16. [PubMed] [Google Scholar]
3. Bhaskar SB. Emergence from anaesthesia: Have we got it all smoothened out? Indian J Anaesth. 2013;57:1–3. [PMC free article] [PubMed] [Google Scholar]
4. Lee C, Jahr JS, Candiotti KA, Warriner B, Zornow MH, Naguib M. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: A comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009;110:1020–5. [PubMed] [Google Scholar]
5. Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005;103:695–703. [PubMed] [Google Scholar]
6. Nicholson WT, Sprung J, Jankowski CJ. Sugammadex: A novel agent for the reversal of neuromuscular blockade. Pharmacotherapy. 2007;27:1181–8. [PubMed] [Google Scholar]
7. Cameron KS, Fletcher L, Clark JK, Zhang MQ, Orbons LPM. Chemical chelation as a novel method of NMB reversal characterization of the Org 25969 NMB complex: 15. 2001;18:99. [Google Scholar]
8. Zhang MQ. Drug specific cyclodextrins: The future of rapid neuro-muscular block reversal? Drugs Future. 2003;28:347–54. [Google Scholar]
9. Naguib M. Sugammadex: Another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575–81. [PubMed] [Google Scholar]
10. Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl. 2002;41:266–70. [PubMed] [Google Scholar]
11. Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: Synthesis and structure-activity relationships. J Med Chem. 2002;45:1806–16. [PubMed] [Google Scholar]
12. Tarver GJ, Grove SJ, Buchanan K, Bom A, Cooke A, Rutherford SJ, et al. 2-O-substituted cyclodextrins as reversal agents for the neuromuscular blocker rocuronium bromide. Bioorg Med Chem. 2002;10:1819–27. [PubMed] [Google Scholar]
13. Cameron KS, Clark JK, Cooper A, Fielding L, Palin R, Rutherford SJ, et al. Modified gamma-cyclodextrins and their rocuronium complexes. Org Lett. 2002;4:3403–6. [PubMed] [Google Scholar]
14. Epemolu O, Bom A, Hope F, Mason R. Reversal of neuromuscular blockade and simultaneous increase in plasma rocuronium concentration after the intravenous infusion of the novel reversal agent Org 25969. Anesthesiology. 2003;99:632–7. [PubMed] [Google Scholar]
15. Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, et al. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: Efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106:935–43. [PubMed] [Google Scholar]
16. Yang LP, Keam SJ. Sugammadex: A review of its use in anaesthetic practice. Drugs. 2009;69:919–42. [PubMed] [Google Scholar]
17. Schering-Plough; 2008. Jul 29, BRIDION(R) (Sugammadex) Injection-First and Only Selective Relaxant Binding Agent-Approved in European Union. [Google Scholar]
18. Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: A comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104:569–74. [PubMed] [Google Scholar]
19. Kovac AL. Sugammadex: A selective relaxant binding agent for neuromuscular block reversal. Formulary. 2009;44:13–21. [Google Scholar]
20. Schering-Plough (press release); 2008. Aug 01, US FDA Issues Action Letter for Sugammadex. [Google Scholar]
21. Naguib M, Mohamed, Brull, Sorin J. Update on neuromuscular pharmacology. Curr Opin Anaesthesiol. 2009;26:874–84. [Google Scholar]
22. Staals LM, Snoeck MM, Driessen JJ, Flockton EA, Heeringa M, Hunter JM. Multicentre, parallel-group, comparative trial evaluating the efficacy and safety of sugammadex in patients with end-stage renal failure or normal renal function. Br J Anaesth. 2008;101:492–7. [PubMed] [Google Scholar]
23. Dahl V, Pendeville PE, Hollmann MW, Heier T, Abels EA, Blobner M. Safety and efficacy of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in cardiac patients undergoing noncardiac surgery. Eur J Anaesthesiol. 2009;26:874–84. [PubMed] [Google Scholar]
24. Hemmerling TM, Russo G, Bracco D. Neuromuscular blockade in cardiac surgery: An update for clinicians. Ann Card Anaesth. 2008;11:80–90. [PubMed] [Google Scholar]
25. Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: A randomized comparison with neostigmine. Anesthesiology. 2008;109:816–
24. [PubMed] [Google Scholar]
26. Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Hunter JM, et al. Sugammadex for reversal of neuromuscular block after rapid sequence intubation: A systematic review and economic assessment. Br J Anaesth. 2010;105:568–75. [PMC free article] [PubMed] [Google Scholar]
27. Bajaj P. Reversal by sugammadex. Indian J Anaesth. 2009;53:399–400. [PMC free article] [PubMed] [Google Scholar]
28. Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104:555–62. [PubMed] [Google Scholar]
29. Abrishami A, Ho J, Wong J, Yin L, Chung F. Cochrane corner: Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Anesth Analg. 2010;110:1239. [PubMed] [Google Scholar]
30. Miller RD. Sugammadex: An opportunity to change the practice of anesthesiology? Anesth Analg. 2007;104:477–8. [PubMed] [Google Scholar]
31. Suy K, Morias K, Cammu G, Hans P, van Duijnhoven WG, Heeringa M, et al. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106:283–8. [PubMed] [Google Scholar]
32. Eleveld DJ, Kuizenga K, Proost JH, Wierda JM. A temporary decrease in twitch response during reversal of rocuronium-induced muscle relaxation with a small dose of sugammadex. Anesth Analg. 2007;104:582–4. [PubMed] [Google Scholar]
33. Ledowski T, Hillyard S, O’Dea B, Archer R, Vilas-Boas F, Kyle B. Introduction of sugammadex as standard reversal agent: Impact on the incidence of residual neuromuscular blockade and postoperative patient outcome. Indian J Anaesth. 2013;57:46–51. [PMC free article] [PubMed] [Google Scholar]
34. Tayal G, Kundra S, Grewal A. Sugammadex-New neuromuscular block reversal. J Anaesth Clin Pharmacol. 2008;24:211–4. [Google Scholar]
35. Naguib M, Magboul MM. Adverse effects of neuromuscular blockers and their antagonists. Drug Saf. 1998;18:99–116. [PubMed] [Google Scholar]
36. Duvaldestin P, Kuizenga K, Saldien V, Claudius C, Servin F, Klein J, et al. A randomized, dose-response study of sugammadex given for the reversal of deep rocuronium- or vecuronium-induced neuromuscular blockade under sevoflurane anesthesia. Anesth Analg. 2010;110:74–82. [PubMed] [Google Scholar]
);You may like
Lastest Price from SUGAMMADEX manufacturers

US $0.00/Kg/Bag2023-11-15
- CAS:
- 343306-71-8
- Min. Order:
- 1KG
- Purity:
- 99%min HPLC
- Supply Ability:
- 100kgs

US $120.00/kg2023-06-26
- CAS:
- 343306-71-8
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 500kg/month