Songorine--a diterpenoid alkaloid of the genus Aconitum
Preclinical Studies of Alkaloids
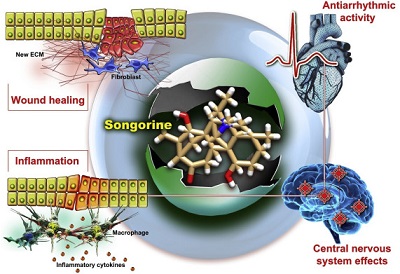
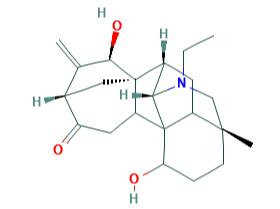
Alkaloids are well-studied secondary metabolites, with recent preclinical studies evidencing that many of them exhibit anti-cancer, anti-depressant, anti-nociceptive, anti-inflammatory, anti-pyretic, anti-platelet, anti-oxidant, and anti-bacterial properties. Aconitum is a genus rich of diverse alkaloids. More than 450 alkaloids have been identified in a variety of species. Songorine is a C20 diterpenoid alkaloid and 12-keto analog of napelline, isolated from Aconitum soongaricum and was associated with a heterogeneous panel of biological functions.
In preclinical models, biological activities were associated with songorine administration. The most significant data found in the scientific literature were evaluated in order to summarize the potential clinical utility of songorine in a diverse spectrum of pathologies and conditions. Songorine and its derivatives have many pharmacological effects including anti-arrhythmic, anti-cardiac-fibrillation, excitation of synaptic transmission, anxiolytic effects, anti-nociceptive, anti-inflammatory, anti-arthritis effects, and a regenerative effect in a skin excision wound animal model. Pharmacokinetic studies revealed a favorable biodistribution in terms of spatial and temporal parameters. Despite its outstanding pharmacotherapeutic potential, songorine has never been tested in clinical trials. Therefore, further evaluation is required to better evaluate its clinical utility.
Bioactivity Study of Songorine
Electrophysiological effects of songorine was investigated in rat hippocampal slices. Songorine (10 – 100 mM) evoked a concentration-dependent increase in the amplitude of the orthodromic population spike and in the slope of the field e.p.s.p. The enhancement was long-lasting and was not reversed by up to 90 min of washout. Songorine failed to affect size and shape of the presynaptic fiber spike which represents the compound action potential of the Schaffer collaterals. This indicates that enhancement of the synaptic response is no consequence of an increased afferent excitability. The antidromically evoked population spike was not affected by songorine at concentrations up to 100 mM suggesting that the enhancement of the orthodromic population spike and of the field e.p.s.p. was not due to an increase in pyramidal cell excitability. The input-output curve for the postsynaptic population spike was shifted to the left implying that a presynaptic fiber spike of the same size elicited a larger postsynaptic response, indicating a decrease in threshold for generation of the population spike. The songorine-evoked increase in excitability was not affected by the NMDA receptor antagonist, DAP5. However, the effect of songorine was completely abolished by the selective dopamine D2 receptor antagonist sulpiride (0.1 mM) as well as by haloperidol (10 mM) and was mimicked by application of the dopamine releaser, amantadine (100 mM). In contrast, the selective D1 receptor antagonist, SCH23390, did not block the action of songorine. The results indicate that the plant alkaloid songorine enhances excitatory synaptic transmission which may be due to an agonistic action at D2 receptors.
References
[1]. Khan, H.; Mohammad Nabavi, S.; Sureda, A.; et al. Therapeutic potential of songorine, a diterpenoid alkaloid of the genus Aconitum, European J. Medicinal Chem., 2018, 153, 29-33.
[2] Ameri, A. Effects of the Aconitu malkaloid songorine on synaptic transmission and paired-pulse facilitation of CA1 pyramidal cells in rat hippocampal slices, British J. Pharmacolo., 1998, 125, 461-468.
);