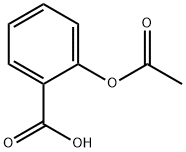
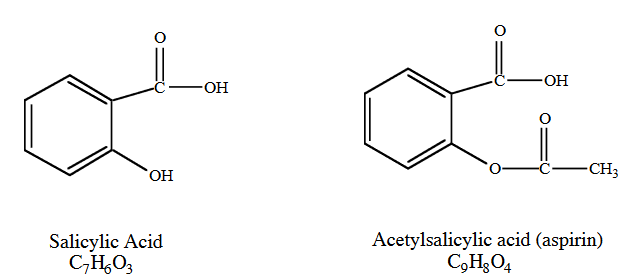
Side effects of Aspirin
The use of aspirin to treat fever dates back to antiquity with the use of willow bark and leaves. Ever since the active component of willow bark was isolated and marketed in 1899, it has remained one of the most popular medications for the treatment of fever, pain, and inflammation. Aspirin is consistently listed among the agents most often involved in human exposures, and is considered the causative agent in scores of deaths each year.

Uses
Aspirin is used as an analgesic, antipyretic, and antiinflammatory agent.
Mechanism of Toxicity
As in humans, the environmental fate of acetylsalicylic acid is pH dependent. Above pH 5.5, acetylsalicylic acid will be the predominant form seen. Anions generally do not volatilize or undergo adsorption to the extent of their neutral counterparts. Although information is limited, aspirin is considered readily biodegradable and is ultimately mineralized to carbon dioxide and water.
Side effects
The most common adverse effects produced by the Aspirin are GI disturbances. Occult blood loss from the GI tract, peptic ulceration, and rarely, severe GI hemorrhage can occur. Because salicylic acid is highly bound to plasma proteins, it may be displaced by other highly protein-bound drugs such as oral anticoagulants, sulfonylureas, phenytoin, penicillins, and sulfonamides. The Aspirin have greatly reduced effects on blood loss and produce fewer adverse GI effects. In addition, they may be somewhat kidney sparing.
Aspirin may provoke hypersensitivity reactions and prolonged bleeding time in some individuals. Tinnitus, hearing impairment, blurred vision, and lightheadedness are indicators of toxic dosages. The use of aspirin in conjunction with any other NSAID is not recommended because of the lack of evidence that such combinations increase efficacy and because of the increased potential for an adverse reaction. Salicylates are contraindicated in children with febrile viral illnesses because of a possible increased risk of Reye’s syndrome.
Environmental Fate
Anion gap metabolic acidosis occurs from a buildup of organic acids as well as the uncoupling of oxidative phosphorylation, which results in an imbalance in ATP consumption and production, resulting in a net buildup of hydrogen ions.
Therefore, aspirin often causes a mixed acid–base status. Furthermore, the uncoupling of oxidative phosphorylation results in failure to produce ATP despite increased oxygen utilization, which leads to heat production and hyperthermia. Aspirin interferes with glucose metabolism and gluconeogenesis, and can cause profound decreases in cerebrospinal fluid glucose concentrations despite normal blood glucose concentrations.
You may like
Related articles And Qustion
Lastest Price from Acetylsalicylic acid manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 50-78-2
- Min. Order:
- 25KG
- Purity:
- 99.5%-101%
- Supply Ability:
- 500KG

US $1.00/KG2025-04-21
- CAS:
- 50-78-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt