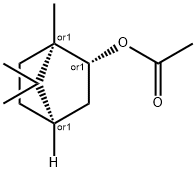
Method for preparing isoborneol by continuous saponification of isobornyl acetate
The invention discloses a method for preparing isoborneol by continuous saponification of isobornyl acetate. The method comprises the following steps: saponifying sodium hydroxide and isobornyl acetate as raw materials, rectifying, layering, washing with water, crystallizing and the like to prepare white isoborneol crystals. The saponification reaction of isobornyl acetate can be continuously carried out in an oscillatory flow reactor, a static mixer or an ultrasonic mixer; a polar solvent is added in the reaction, so that the saponification reaction is heterogeneously carried out, the reaction time can be shortened, the reaction temperature can be reduced, the conversion rate of isobornyl acetate is more than 99%, and the product yield is more than 95%. The method is low in production energy consumption and high in product yield.
Description
One kind of acid isobornyl continuously prepared isoborneol saponification method

FIELD
[0001] The present invention belongs to the field of chemical industry, particularly relates to a method for continuously isobornyl acetate saponified prepared isoborneol.
Background technique
[0002] Also known isoborneol isobornyl alcohol, a forest chemical products, is the difference borneol epimer, formula C1 (lH 180. Isoborneol has a peculiar smell, flavor and can be used for the medical field, camphor synthetic intermediates.
[0003] synthesis method industrially isoborneol main Direct and indirect two processes. Direct method under acidic catalyst directly to the hydration of camphene isoborneol, the advantage of simplifying the steps, saves raw material, but the relatively low conversion and selectivity. The indirect method is esterification of acetic acid to camphene and isobornyl acetate, then saponified isobornyl acetate generate isoborneol, which process is more mature. Current industrial synthesis of multiple indirect synthesis isoborneol, but a batch operation saponification reaction, a complex having a saponification process, a long reaction time, high energy consumption, lower yield and other shortcomings. Thereby selecting suitable reactor, the co-solvent is a critical indirect method isobornyl acetate saponification reaction efficiency. The present invention provides a higher yield, shorter reaction time, lower energy consumption and production costs, the production method can be achieved isoborneol continuous operation in the industry to meet the needs of modern industrial production.
SUMMARY
[0004] The object of the present invention is to provide a continuous isobornyl acetate saponification process for preparing isoborneol, sodium hydroxide and acetic acid are isobornyl acrylate as raw materials, and prepared for industrial continuous saponification isoborneol. The method of mild reaction conditions, short reaction time, the conversion of the brain acetate, isopropyl ester Long more than 99%, more than 95% yield of the product, and recycling of a polar solvent.
[0005] To achieve the above object, the present invention adopts the following technical solutions: A acetate, isobornyl continuous saponification process for preparing isoborneol, sodium hydroxide and acetic acid are isobornyl prepared starting isoborneol which preparation methods include saponification, distillation, delamination, washing, crystallization step.
[0006] specifically includes the following steps: 1) Stir the mixed aqueous sodium hydroxide with a polar solvent to prepare a polar solution; 2), respectively, and isobornyl acetate Step 1) from the polar solution saponification reaction end of the pump into the filter, saponification; 3) respectively, a nonpolar solvent and water from the saponification reactor into the other end of the pump, a non-polar solvent solution with the homogeneous saponification [J, even after mixing with water from the saponification reactor outlet taken out of the reaction solution; 4) pumped into the reaction distillation column, normal pressure distillation of the reaction liquid recovered polar solvent; polar solvent recovery resulting from the overhead can be reused , the remaining solution was taken out from the column bottom; 5) of the recovery column Tsuen layered solution, the oil phase containing isoborneol mixture, the aqueous phase is sodium acetate aqueous solution; 6) the oil phase was washed with water recovery , standing layer, washed repeatedly with water until the eluate was neutral pH; 7) by evaporation crystallization method, after washing the oil phase crystallization, filtration to give white crystals isoborneol.
The [0007] Step 1) molar ratio of aqueous sodium hydroxide solution with a polar solvent is 1: 1 to fly; mass fraction of the aqueous sodium hydroxide solution is 3 (Γ46%; the polar solvent include acetone, methanol, acetonitrile, isopropanol, dioxane.
[0008] Step 2) the acetic acid isopropyl ester brain Lung purity> 97%.
[0009] Step 2) the molar ratio of acetic acid and sodium hydroxide isobornyl saponification is 1: 105 ~ 125.
[0010] Step 2) saponification of the oscillatory flow reactor is a reactor, a static mixer or ultrasonic mixer.
[0011] Step 2) The reaction time was saponification reaction 1 (T60 min, the reaction temperature is 6 (Tl00 ° C.
Molar ratio [0012] Step 3) isobornyl acetate, a nonpolar solvent and water is 1: 2 wide: two fly; the non-polar solvent is a benzene solvent.
[0013] A significant advantage of the present invention is that: (1) The present invention employs a polar solvent in the saponification process, the two phases into a homogeneous saponification reaction, the saponification reaction conditions more moderate, and the reaction time from saponification 8~10h reduced to l (T60min, greatly shorten the reaction time.
[0014] (2) a saponification reaction of the present invention employs oscillating flow mixer, a static mixer or an ultrasonic mixer, a continuous operation can achieve saponification.
[0015] (3) the use of the present invention were prepared isoborneol, isoborneol its acetate ester conversion of the brain more than 99%, more than 95% product yield.
[0016] (4) Preparation process of the invention was added to the distillation step, a polar solvent can be recovered and reused, reducing waste emissions, facilitate continuous production.
BRIEF DESCRIPTION
[0017] FIG. 1 is a process flow diagram of Example 1 and Example 2 Preparation of continuous saponification Isoborneol embodiment; wherein: 1- aqueous sodium hydroxide inlet, a polar solvent inlet 2-, 3-acetate, isopropyl Long brain inlet ester, 4- nonpolar solvent feed inlet, a feed water inlet 5-, 6- polar solvent outlet, water inlet 7-, 8- isoborneol discharge opening; V-101- polar solution storage tank, V-102- reservoir, V-103- delaminator, V-104- washing tank, P-101- oscillating pumps, R-101- saponification reactor, T-101- distillation column, D-101- crystallizer.
[0018] FIG. 2 is a process flow diagram for the continuous preparation of 3 isoborneol Example saponification embodiment; wherein: 1- aqueous sodium hydroxide inlet, a polar solvent inlet 2-, 3- isobornyl acetate feed mouth, 4- nonpolar solvent feed inlet, a feed water inlet 5-, 6- polar solvent outlet, water inlet 7-, 8- isoborneol discharge opening; V-101- polar solution reservoir tank, V-102- reservoir, V-103- delaminator, V-104- water reactor, R-101- saponification reactor, T-101- fractionator, D-101- crystallizer.
Detailed ways
[0019] In order to make the content easier understanding of the present invention, the following embodiment with reference to specific embodiments further illustrate the technical solutions of the present invention, but the present invention is not limited thereto.
[0020] Example 1. An acetic acid isobornyl continuous method of preparing saponified isoborneol, includes the following steps: 1) the mass fraction of 46% aqueous sodium hydroxide solution (1) with a polar solvent such as acetone (2 ) was pumped into the polar solution storage tank V-101, stirred uniformly mixed to prepare a polar solution; the molar ratio of acetone and aqueous sodium hydroxide is 1: 3; 2) press isobornyl acetate hydroxide of 1 molar ratio of sodium: polar solution pump 12, respectively, isobornyl acetate, (3) step 1) of FIG oscillatory flow into R-101 1 reactor, the circulating water temperature was stabilized to 80 ° C, while shaking open pump P-101, adjusting the oscillation frequency of 4Hz, oscillation amplitude 0. 03m, saponification 30 min; the isobornyl acetate purity of 97.8%; 3) to be within the reactor feed solution when the height of non-polar solvent inlet height, according isobornyl acetate, and the molar ratio of non-polar solvent to water is 1: 5 1: 5, respectively, nonpolar solvent such as xylene (4), and water (5 ) was pumped into the upper portion of the reactor, xylene and the solution after the saponification reaction, water mixed reaction liquid taken out from the top of the reactor, V-102 was pumped into the reservoir ; 4) V-102 is pumped into the reaction distillation column T-101, a normal pressure distillation of the reaction liquid recovered in a polar solvent, column top temperature of 55 ° C, column temperature 70 ° C tower bottom temperature of 80 ° C; the resulting polar solvent (6) taken out from the column top, the remaining solution was taken out from the tower bottom, is pumped into the stratified stratified V-103, the resulting oil phase containing isoborneol mixture, the aqueous phase is sodium acetate aqueous solution; 5) the oil phase was pumped into the water recovery tank V-104, and pumped into the water (7) was separated out was washed repeatedly washed with water until the eluate was neutral pH ; 6) the oil phase after washing with water was pumped into the crystallizer D-101, crystallization by evaporation, subjected to crystallization, filtration to give white crystals isoborneol (8).
[0021] The gas chromatograph of isobornyl acetate, isoborneol in brain analyzed, this reaction product obtained isobornyl purity 95.8%, bornyl acetate, isopropyl acetate conversion 99.3% , isobornyl yield of 95.4%.
[0022] 2 one kind of isobornyl acetate embodiment a continuous process isoborneol saponified preparation includes the following steps: 1) the mass fraction of 40% aqueous sodium hydroxide solution (1) with a polar solvent of methanol (2 ) was pumped into the polar solution storage tank V-101, stirred uniformly mixed to prepare a polar solution; molar ratio of the aqueous sodium hydroxide and methanol is 1: 14; 2) press and isobornyl acetate molar ratio of sodium hydroxide to 1: polar solution pump 115, respectively, isobornyl acetate, (3) step 1) of FIG oscillatory flow into R-101 1 reactor, the circulating water temperature stabilizing to 70 ° C, while shaking open pump P-101, adjusting the oscillation frequency of 3Hz, oscillation amplitude 0. 05m, saponification 25 min; purity of the acid was isobornyl 97.3%; 3) until the reactor when the starting material liquid reaches the height of the height of non-polar solvent inlet, a molar ratio isobornyl acetate, a nonpolar solvent and water is 1: 1 3: 2, respectively, a non-polar solvent of toluene (4), and water ( 5) pumped into the reactor from an upper portion of the toluene solution after the saponification reaction, even after mixing with water taken from the top of the reactor out of the reaction solution was pumped into the reservoir V-102; 4) The V-102 was pumped into the reaction distillation column T-101, a normal pressure distillation of the reaction liquid recovered in a polar solvent, column top temperature of 55 ° C, column temperature of 70 ° C, the column bottom temperature 80 ° C; the resulting polar solvent (6) taken out from the column top, the remaining solution was taken out from the tower bottom, is pumped in layered stratified V-103, containing isoborneol mixing the resulting oily phase solution, the aqueous phase is sodium acetate aqueous solution; 5) the oil phase was pumped into the water recovery tank V-104, and pumped into the water (7) was separated out was washed repeatedly washed with water until the eluate was neutral pH; 6) the oil phase after washing with water was pumped into the crystallizer D-101, crystallization by evaporation, subjected to crystallization, filtration to give white crystals isoborneol (8).
[0023] The gas chromatograph of isobornyl acetate, isoborneol in brain analyzed, this reaction product obtained isobornyl purity 96.15%, conversion of the acid isobornyl ester 99.5% , isobornyl yield of 96.1%.
[0024] 3 one kind isobornyl acetate embodiment continuous saponification process for preparing isoborneol, includes the following steps: 1) the mass fraction of 30% aqueous sodium hydroxide solution (1) with the polar solvent isopropyl alcohol (2) polar solution is pumped into storage tank V-101, stirred uniformly mixed to prepare a polar solution; molar ratio of the aqueous sodium hydroxide solution and isopropyl alcohol is 1: 6; 2) by acid isoborneol molar ratio of ester to sodium hydroxide is 1: 1 1, respectively, isobornyl acetate, (3) a static mixer and R-101 2 step 1) in FIG polar solution is pumped into the water temperature stable to 60 ° C, saponification 10 min; purity of the brain acid isopropyl ester was Lung 97.1%; 3) to be the height of the reactor feed solution inlet height nonpolar solvent, isobornyl acetate by the molar ratio of non-polar solvent and water is 1: 2: 6, respectively, the non-polar solvent diethylbenzene (4) water and (5) from the reactor and pumped into an upper portion of the solution after the saponification and diethyl benzene, even after mixing with water taken from the top of the reactor out of the reaction solution was pumped into the reservoir V-102; 4) the V-102 was pumped into the reaction distillation column T-101, the use of atmospheric distillation The reaction liquid recovered in a polar solvent, column top temperature of 55 ° C, column temperature of 70 ° C, column bottom temperature 80 ° C; the resulting polar solvent (6) taken out from the column top, the remaining solution was collected from the column reactor out, pumped into the V-103 is a layered, stratified resulting oil phase containing isoborneol mixture, the aqueous phase is sodium acetate aqueous solution; 5) the oil phase was pumped into the water recovery tank V-104, and after pumped into the water (7) was separated out was washed repeatedly washed with water until the eluate was neutral pH; 6) the oil phase after washing with water was pumped into the crystallizer D-101, by evaporation crystallization method, crystallization from, filtered to give a white crystalline isoborneol (8).
[0025] The gas chromatograph of isobornyl acetate, isoborneol in brain analyzed, this reaction product obtained isobornyl purity 95.03%, isobornyl acetate conversion 99.2% , isobornyl yield of 95.2%.
[0026] The foregoing is only preferred embodiments of the present invention, where the application under this invention, modifications and alterations made to the scope of the patent, also belong to the scope of the present invention.
);You may like
Related articles And Qustion
See also
Lastest Price from Isobornyl acetate manufacturers

US $10.00/kg2024-04-24
- CAS:
- 125-12-2
- Min. Order:
- 1kg
- Purity:
- 99.6%
- Supply Ability:
- 100000

US $0.00/Kg/Drum2024-04-24
- CAS:
- 125-12-2
- Min. Order:
- 180KG
- Purity:
- 98
- Supply Ability:
- 10000