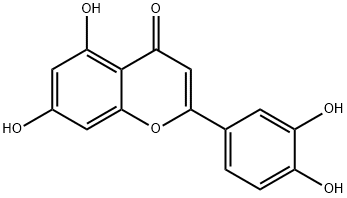
Luteolin - A flavone common in plants
Luteolin, 3′,4′,5,7-tetrahydroxyflavone, is with a yellow crystalline appearance. Belonging to the flavone group of flavonoids, luteolin has a C6-C3-C6 structure and possesses two benzene rings (A, B), a third, oxygen-containing (C) ring, and a 2−3 carbon double bond. Luteolin also possesses hydroxyl groups at carbons 5, 7, 3’, and 4’ positions [1]. The hydroxyl moieties and 2−3 double bond are important structure features in luteolin that are associated with its biochemical and biological activities. As in other flavonoids, luteolin is often glycosylated in plants, and the glycoside is hydrolyzed to free luteolin during absorption. Some portion of luteolin is converted to glucuronides when passing through the intestinal mucosa. Luteolin is heat stable and losses due to cooking are relatively low [2].

Luteolin are found widely existing in many types of plants including fruits, vegetables, and medicinal herbs. Plants rich in luteolin have been used in Chinese traditional medicine for treating various diseases such as hypertension, inflammatory disorders, and cancer. Having multiple biological effects such as anti-inflammation, anti-allergy and anticancer, luteolin functions as either an antioxidant or a pro-oxidant biochemically. The biological effects of luteolin could be functionally related to each other. For instance, the anti-inflammatory activity may be linked to its anticancer property. Luteolin's anticancer property is associated with the induction of apoptosis, and inhibition of cell proliferation, metastasis and angiogenesis. Furthermore, luteolin sensitizes cancer cells to therapeutic-induced cytotoxicity through suppressing cell survival pathways such as phosphatidylinositol 3′-kinase (PI3K)/Akt, nuclear factor kappa B (NF-κB), and X-linked inhibitor of apoptosis protein (XIAP), and stimulating apoptosis pathways including those that induce the tumor suppressor p53. These observations suggest that luteolin could be an anticancer agent for various cancers. Furthermore, recent epidemiological studies have attributed a cancer prevention property to luteolin [3].
Vegetables and fruits such as celery, parsley, broccoli, onion leaves, carrots, peppers, cabbages, apple skins, and chrysanthemum flowers are luteolin rich [4]. Plants rich in luteolin have been used as Chinese traditional medicine for hypertension, inflammatory diseases, and cancer. The pharmacological activities of luteolin could be functionally related to each other. For instance, the anti-inflammatory effect of luteolin also may be linked to its anticancer function. The anticancer property of luteolin is associated with inducing apoptosis, which involves redox regulation, DNA damage, and protein kinases in inhibiting proliferation of cancer cells and suppressing metastasis and angiogenesis. Furthermore, luteolin sensitizes a variety of cancer cells to therapeutically induced cytotoxicity through suppressing cell survival pathways and stimulating apoptosis pathways. Notably, luteolin is blood-brain barrier permeable, rendering it applicable to the therapy of central nerve system diseases, including brain cancer [5]. Furthermore, recent studies have attributed a cancer prevention potential to luteolin. In this review, we summarize recent progress in luteolin researches. Particularly, we focus on the roles and molecular mechanisms underlying luteolin's anticancer property.
References
[1] J.A. Ross, C.M. Kasum, Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr, 2002; 22:19–34.
[2] L. Le Marchand, Cancer preventive effects of flavonoids--a review. Biomed. Pharmacother, 2002; 56:296–301.
[3] Y. Lin, R. X. Shi, X. Wang, H. M. Shen, Luteolin, a flavonoid with potentials for cancer prevention and Therapy. Curr Cancer Drug Targets, 2008; 8(7): 634–646.
[4] K. H. Miean, S. Mohamed, Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem., 49:3106–3112.
[5] C. J. Wruck, M. Claussen, G, Fuhrmann, L. Romer, A. Schulz, T. Pufe, V. Waetzig, M. Peipp, T. Herdegen, M. E. Gotz. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural Transm. Suppl., 2007; 72:57–67.
You may like
Related articles And Qustion
See also
Lastest Price from Luteolin manufacturers

US $1200.00-1150.00/ton2025-09-26
- CAS:
- 491-70-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00/KG2025-09-18
- CAS:
- 491-70-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month