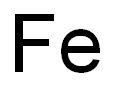Iron - Drug Discovery and Legand
Iron has been known to mankind from early civilization. In fact, a period of history, the “iron age,” is named for the widespread use of this metal. For almost a thousand years, it remained as the single most-used metal, and its use in mechanization made the industrial revolution possible. Iron, after oxygen, silicon and aluminum, is the fourth most abundant element in the earth’s crust. It is the prime constituent of earth’s core along with nickel. Its abundance in the crust is 5.63%. Its concentration in the seawater is about 0.002mg/L. The principal ores of iron are hematite, Fe2O3; pyrite, Fe2S2; ilmenite, FeTiO3; magnetite, Fe3O4; siderite, Fe2CO3; and limonite [FeO(OH)]. It also is found in a number of minerals, such as corundum, as an impurity. It also is found in meteorites.

Iron occurs in every mammalian cell and is vital for life processes. It is bound to various proteins and found in blood and tissues. The iron-porphyrin or heme proteins include hemoglobin, myoglobin and various heme enzymes, such as cytochromes and peroxidases. Also, it occurs in non heme compounds, such as ferritin, siderophilin, and hemosiderin. Hemoglobin, found in the red blood cells, is responsible for transport of oxygen to the tissue cells and constitutes about two-thirds (mass) of all iron present in the human body. An adult human may contain about 4 to 6 grams of iron.
Iron appears to be the first mineral known to have been administered by mouth for therapeutic purposes. One of the oldest Greek legends tells how Melampus, a shepherd endowed with magical powers, cured the infertility of Iphiclus, King of Phylacea, by giving him wine in which rust scrapings from a knife had been steeped. Ferric oxide occurs naturally as a mineral, red haematite, and in the form of rust. Throughout recorded history, the astringent properties of ferric oxide have been exploited in various ways, such as in preparations for soothing the eye. It is mentioned in the Ebers Papyrus as an ingredient of a paste for the treatment of eye disease,2 while haematite in ox fat was applied to the eye by Babylonian physicians to alleviate photobia.3 The Hippocratic writings described the use of iron salts to stem bleeding, possibly resulting from the belief that battle wounds were best healed by application of the agent that caused them. Dioscorides noted that rust can stem uterine haemorrhage and also prevent conception,4 while evidence of iron therapy for debility during Roman times has been obtained from excavations in Gloucestershire, England.5 A detailed account of the use of iron by the Greeks and Romans was provided by Nicolas Monardes in the 2nd volume of his Historia Medicinal de las Cosas que se Traen de Nuestras Indias Occidentales . . . , where he cited them for conditions such as the bloody flux (amoebic dysentery), the flux of women (menstruation) and piles.

Lazarus Riverius, a physician to Louis XIV, in 1640 published the 15th volume of his Praxis Medica, a work that was translated into English 15 years later by Culpepper, Cole and Rowland as The Practice of Physic. This recommended steel dissolved in wine for chlorosis, a disease of young women sometimes described as love sickness, but now recognised as iron deficiency anaemia. The selection of an acid wine would have facilitated the oxidation of the iron. In 1681, Thomas Sydenham specified iron as the cure for chlorosis.5 He considered this to be a manifestation of hysteria caused by a weakness of the animal spirits, which could be cured by strengthening the blood as this was the origin of these spirits. He advised that the patient should be bled before starting a 30 day course of pills containing steel filings and extract of wormwood, each dose being followed by a draught of wormwood wine.
The Parisian apothecary Pierre Pomet (1658–1699) wrote his Histoire Generale des Drogues, which was translated into English in 1712 as the Compleat History of Drugs, with additions by the Apothecary at the Jardin du Roi in Paris, Nicolas Le´mery, and the medical botanist, Joseph Pitton de Tounefort. This recommended crocus martis, which is ferric oxide, for chlorosis and dropsy. A year after the publication of the English edition, Le´mery and Geoffy provided a rational basis for iron therapy by demonstrating the presence of iron in ash prepared from blood.6

The first use of ferrous sulfate was by the French physician Pierre Blaud (1774–1858).7 This reduced form of iron occurs naturally as a hydrate in several minerals, but can readily be prepared by dissolving iron in sulfuric acid. In 1832, Blaud reported that he had cured 30 cases of chlorosis within 10 to 30 days by administering pills containing 320 mg each of ferrous sulfate and potassium carbonate. He believed earlier failures of iron therapy were due to the underdosing of patients. The amount of ferrous sulfate he administered was gradually increased until his patients were taking 12 pills daily, a dose that would now be considered excessive as 200mg two or three times a day is recommended. Blaud’s nephew, a pharmacist, sold the ‘veritable pills of Doctor Blaud’ throughout the world.8 Many similar products appeared on the market and other ferrous salts were introduced, such as the carbonate, citrate, fumarate, gluconate, iodide, lactate, phosphate and succinate.
You may like
Related articles And Qustion
See also
Lastest Price from Iron manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 7439-89-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton

US $1300.00-1200.00/tons2025-03-07
- CAS:
- 7439-89-6
- Min. Order:
- 10tons
- Purity:
- 99%
- Supply Ability:
- 1000tons