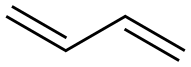Butadiene:Mechanism of Toxicity
Butadiene is produced during the combustion of organic matter. Significant amounts of butadiene are released to the environment from both natural and anthropogenic sources such as forest fires, gasoline and diesel engine exhaust, and wood space heating. It is also a component of cigarette smoke. Industrially, butadiene is most commonly produced as a coproduct of ethylene manufacture in which hydrocarbon feedstocks are cracked with steam and butadiene is removed from the resulting crude C4 stream by liquid–liquid extraction with solvents.

Uses
Butadiene is a reactive monomer used in the production of synthetic rubber (60%) and plastics. Styrene–butadiene rubber, polybutadiene rubber, adiponitrile, styrene–butadiene latex, acrylonitrile–butadiene–styrene resins, and nitrile rubber are used in the manufacture of tires, nylon products, plastic bottles and food wraps, molded rubber goods, latex adhesives, carpet backing and pads, shoe soles, and medical devices. There are no direct consumer uses of butadiene and unreacted monomer is not expected to be present in polymers and plastics made from butadiene.
Environmental Fate
Butadiene is a gas under normal environmental conditions with limited water solubility (735 mg l-1 at 25°C). Butadiene released to the atmosphere will remain there with very small amounts being distributed to water and soil. In air, butadiene will be removed by reaction with photochemically produced hydroxyl radicals (5.6-h half-life), nitrate radicals (15-h half-life), and ozone (1.5-day half-life). When released to water, butadiene will be removed by volatilization to air (Henry’s law constant of 7460 Pam3 mol-1), biodegradation (aerobic half-life of 15 days), and reaction with singlet oxygen. Based on its estimated organic carbon partition coefficient (Koc of 288), butadiene will not exhibit significant adsorption to soil or suspended particulate matter; its biodegradation half-life in soil is estimated to be 30 days. Due to volatilization to air and degradation in soil, butadiene is not expected to leach to groundwater. As butadiene is readily metabolized, it is not expected to pose a significant bioaccumulation hazard.
Mechanism of Toxicity
The carcinogenic potential of butadiene in rodents is thought to reflect the metabolism of butadiene to DNA-reactive metabolites resulting in genetic alterations in protooncogenes and/or tumor suppressor genes. Mechanistic data suggest that the higher carcinogenic potency of butadiene in mice versus rats is primarily due to the higher body burden in mice of DEB, considered the most mutagenic butadiene metabolite (see Genotoxicity). This is supported by the observations that carcinogenicity tests with EB were equivocal, while DEB was carcinogenic in mice and rats when administered via multiple routes of exposure. Although the mechanism by which butadiene causes noncancer effects is not well understood, there is strong evidence that ovarian atrophy in mice, the most sensitive noncancer endpoint, is mediated by DEB.
Related articles And Qustion
See also
Lastest Price from 1,3-Butadiene manufacturers

US $1.00/KG2025-04-21
- CAS:
- 106-99-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-03-21
- CAS:
- 106-99-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month