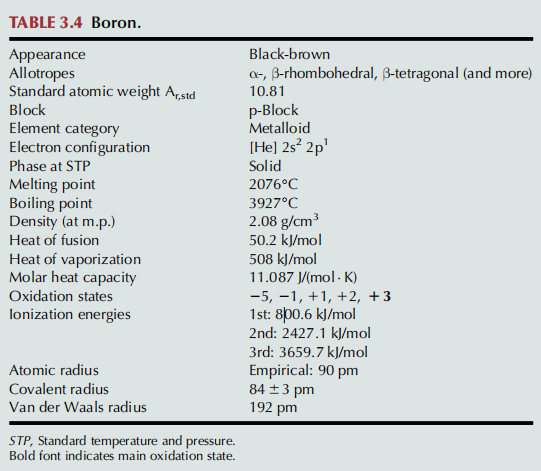
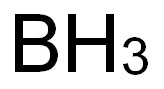Boron——Sources of Exposure & Industrial Hygiene
First aid:
Eye contact: Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 min. Get medical attention if irritation occurs.
Skin Contact: Wash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops.
Inhalation: If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention.
Ingestion: Do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband.
Target organ(s): Skin, eyes, lungs, systemic toxicant
Occupational exposure limits: Boron oxide, total dust (8 h TWA): 15 mg/m3 (OSHA)
Boron trifluoride (ceiling): 3 mg/m3 (OSHA) Reference values: Boron and boron compounds: RfC N/A (EPA); RfD 0.2 mg/kg/day (EPA); Inhalation MRL 0.3 mg/m3 (ATSDR); OralMRL 0.2 mg/kg/day (ATSDR)
Risk/safety phrases: Skin irritant; eye irritant; corrosive
SOURCES OF EXPOSURE
Occupational
The NIOSH estimates that the number of workers potentially exposed to boron increased from 6500 in the early 1970s to 35,600 in the early 1980s (NOHS, 1989; NOES, 1989). Sittig (1985) reports that NIOSH estimated that the numbers of workers potentially exposed to borax, boron oxide, and boron trifluoride are 2,490,000; 21,000; and 50,000, respectively.
Workers in the industries of manufacture of glass wool, fiberglass and other glass products, cleaning and laundry products, fertilizers, pesticides, and cosmetics may be exposed to boron compounds (Jensen, 2009; Stokinger, 1981). Workers exposed to boron may absorb measurable quantities; end-of-shift boron concentrations in blood and urine of 0.11–0.26 μg/g and 3.16–10.72 μg/mg creatinine, respectively, have been reported in workers at a facility where borax is packaged and shipped (Culver et al., 1994). In occupational settings where higher exposures exist without exposure mitigation, boron exposure can be substantially higher. Workers from Kuandian City, China employed in boron mines or processing plants producing borax or boric acid had total daily boron measurements (in food, fluids, and personal air) averaging 41.2 mg/day for workers in the boron industry, 4.3 mg/day in the surrounding community, and 2.3 mg/day in a control group. Post-shift urine levels of boron measured in the boron industry workers was 499 mg/day, compared to 48.0 mg/day in the control group (Xing et al., 2008).
Large borax mining and refining plants may produce borax dusts in concentrations ranging from 1.1 to 14.6 mg/ m3 for total particulate with a mean boric acid/boron oxide dust concentration of 4.1 mg/m3 for total particulate (Garabrant et al., 1984, 1985). In air samples from U.S. facilities where borax was packaged and shipped, mean dust concentrations have been observed in the range of 3.3–18 mg/m3 (Culver et al., 1994).
Environmental
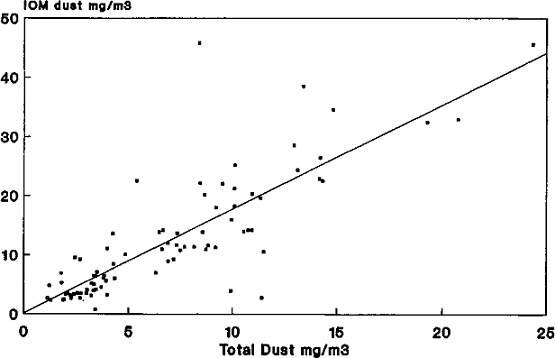
The Relationship of Blood- and Urine-boron to Boron Exposure
Ingestion of boron from food and water is the most frequent route of environmental human exposure. Boron exposure may also occur from some consumer products, including cosmetics, medicines, and insecticides. Some areas of the western United States have natural boron-rich deposits in the soil may also have higher boron exposure.
Rainey et al. (2002) reported mean daily intakes of boron from food and beverage sources for male and female adults to be 1.28 and 1.00 mg, respectively. Daily dietary boron intakes were estimated at 0.75 mg for infants aged 0–6 months and 0.99 mg for infants aged 7–11 months. Daily boron intakes were estimated as 0.86 mg for children aged 1–3 years, 0.80 mg for children aged 4–8 years, 0.90 and 1.02 mg, respectively, for adolescent males aged 9–13 and 14–18 years, and 0.83 and 0.78 mg, respectively, for adolescent females aged 9–13 and 14–18 years.
Boron is fairly ubiquitous in surface water and groundwater; the average surface water boron concentration in the United States is about 0.1 mg/l (Butterwick et al., 1989; EPA, 1986), but concentrations vary greatly, depending on local geologic and anthropogenic sources of boron (Butterwick et al., 1989). Mean boron concentrations in soil in the United States are about 30 mg/kg, with concentrations ranging up to 300 mg/kg (Eckel and Langley, 1988).
In the United States, Canada, and England, concentrations of boron in tap water have been reported in a range of 0.007–0.2 mg/l (Choi and Chen, 1979; Davies, 1990; Waggott, 1969). Ambient air samples have reported boron concentrations from <5 × 10-7 to 8 × 10-5 mg/m3, with an average concentration of 2 × 10-5mg/m3 (Howe, 1998). It has been estimated that approximately 11,600 tons of boron per year are released into the atmosphere as a component of fly ash produced by coal combustion (Bertine and Goldberg, 1971).
INDUSTRIAL HYGIENE
Borates, or oxygen and inorganic boron-containing compounds, are the most common form of boron found in commercial products. This group includes boric acid, boric oxide, and hydrides of boron (boranes). Elemental boron is rare in the workplace and is not regulated because it is inert and has minimal use. Dust is the most common form of exposure and borate dusts should be kept below regulatory levels through appropriate control measures.
REFERENCES
Butterwick, L., de Oude, N., and Raymond, K. (1989) Safety assessment of boron in aquatic and terrestrial environments. Ecotoxicol. Environ. Saf. 17:339–371.
Choi, W.W. and Chen, K.Y. (1979) Evaluation of boron removal by adsorption on solids. Environ. Sci. Technol. 13:189–196.
Cotton, F.A., Wilkinson, G., Murillo, C.A., et al., editors (1999) Boron. In: Advanced Inorganic Chemistry, 6th ed., New York, NY: John Wiley & Sons, Inc., pp. 131–174.
Culver, B.D., Shen, P.T., Taylor, T.H., Lee-Feldstein, A., Anton- Culver, H., and Strong, P.L. (1994) The relationship of bloodand urine-boron to boron exposure in borax-workers and usefulness of urine-boron as an exposure marker. Environ. Health Perspect. 102:133–137.
Davies, K. (1990) Human exposure pathways to selected organochlorines and PCBs in Toronto and Southern Ontario. Adv. Environ. Sci. Technol. 23:525–540.
Dieter, M.P. (1994) Toxicity and carcinogenicity studies of boric acid in male and female B6C3F1 mice. Environ. Health Perspect. 102(Suppl. 7):93–97.
You may like
Related articles And Qustion
See also
Lastest Price from Boron manufacturers

US $10.00-7.00/kg2025-02-13
- CAS:
- 7440-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $0.00-0.00/g2024-09-25
- CAS:
- 7440-42-8
- Min. Order:
- 50g
- Purity:
- 99%
- Supply Ability:
- 60kg