Applications of Triphenylphosphine
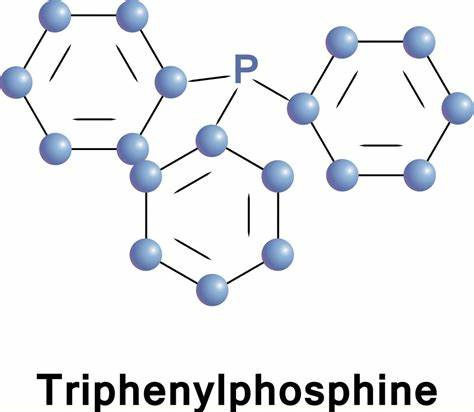
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
Applications
PPh3 is widely used in organic synthesis. The properties that guide its usage are its nucleophilicity and its reducing character.The nucleophilicity of PPh3 is indicated by its reactivity toward electrophilic alkenes, such as Michael-acceptors, and alkyl halides. It is also used in the synthesis of biaryl compounds, such as the Suzuki reaction.
Triphenylphosphine has found widespread use in the reduction of compounds containing the hydroperoxide or endoperoxide functionality to form, depending upon the substrate, alcohols, carbonyl compounds, or epoxides. The primary driving force behind this class of reactions is the formation of the strong P=O bond at the expense of the relatively weak (45–50 kcal mol−1) O–O bond.For example, triphenylphosphine constitutes a mild, selective method for the reductive decomposition of ozonides to ketones and aldehydes.
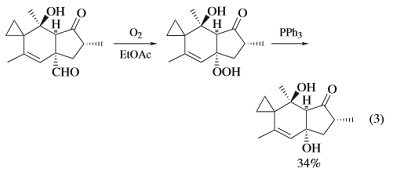
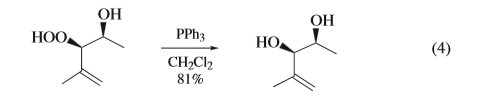
Treatment of hydroperoxides with triphenylphosphine affords the corresponding alcohols in high yields under mild conditions and with retention of configuration at the carbon bearing the peroxide (eqs 3 and 4). The reaction of endoperoxides, on the otherhand,affordsepoxideswithinversionofconfigurationatone of the two carbon atoms Vinylogous endoperoxides reactwithtriphenylphosphinetoaffordtheallylicepoxides.These examples should not imply that reaction of endoperoxides with triphenylphosphine is necessarily facile, since certain endoperoxideshavebeenshowntobeinerttoPPh Nevertheless, thetransformationofthe α-methylene-β-peroxylactonetoafford the β-lactone 16 illustrates the wide scope of the peroxide deoxygenation capability of triphenylphosphine.
The deoxygenation of epoxides affords the corresponding alkenes and results in an inversion in the stereochemistry of substitutents attached to the double bond.While the reaction has been known since the mid 1950s,it has not found widespread use. Triphenylphosphine has found utility as a reducing agent of N-oxides. While several alternative reagents and conditions are available, these often require fairly strong reducing conditions which are incompatible with a wide range of functionality.
In fact, triphenylphosphine-mediated reductions of N-oxides require much more vigorous conditions than do the corresponding reductions of peroxides. For example, reduction of trimethylamineoxidewithtrialkylphosphinesrequiresrefluxinginglacial acetic acid,and the reduction of pyridine N-oxide derivatives is best carried out at temperatures above 200◦C in the absence of solvent. However, it has been found that aromatic amine oxides are reduced in high yield with triphenylphosphine at room temperature with irradiation. While not general, under special conditions triphenylphosphine is also capable of reducing aromatic nitroso and nitro groups.
You may like
Related articles And Qustion
See also
Lastest Price from Triphenylphosphine manufacturers
US $0.00/KG2025-05-21
- CAS:
- 603-35-0
- Min. Order:
- 1000KG
- Purity:
- 99.5
- Supply Ability:
- 20000MT

US $0.00/kg2025-05-21
- CAS:
- 603-35-0
- Min. Order:
- 1000kg
- Purity:
- 99.5
- Supply Ability:
- 20000mt
![174063-87-7 1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene; Preparation](/NewsImg/2023-04-07/6381648507798059725928387.jpg)

![174063-87-7 1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene; Preparation](https://www.chemicalbook.com/NewsImg/2019-11-7/2019117142424284.jpg)