Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine
What is Triphenylphosphine?
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the chemical formula P(C6H5)3, often abbreviated as PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds because it is a potent reducing agent and neutral ligand. At room temperature, PPh3 crystals are relatively stable and colourless in air. PPh3 is insoluble in water; slightly soluble in petroleum ether and alcohol, soluble in xylene, toluene, acetone, carbon tetrachloride and ether.
Convenient Synthesis of Triphenylphosphine Sulfide from
Sulfur and Triphenylphosphine
Although the direct reaction of triphenylphosphine with sulfur leading to triphenylphosphine sulfide was known, this reaction was described to proceed slowly at rt
or require heating at high temperatures. Very recently, the rotary ball mill technique (at
400 rpm) was applied to this reaction to lower the reaction temperature to rt with shortened
reaction time (4 h). However, in this case, although solvents were not used during the
reaction, extraction of the product from the apparatus required a significant amount of
solvent. It should also be noted that although this technique could be useful in some cases,
it is not suitable as a common synthetic method for any synthetic organic laboratory due
to the high price of the apparatus (varying from many thousands to tens of thousands of
dollars for typical laboratory models).
In this context, the simplification of reaction conditions by lowering the solvent amounts, using standard and inexpensive laboratory glassware and purifying the obtained products by simple filtration and washing should be an excellent approach to valorize and promote the widespread application of the developed synthetic procedure.
Herein we report a very rapid reaction of a stoichiometric mixture of triphenylphosphine with sulfur (reaction time less than 1 min) under very simple conditions of shaking.
During the course of our study on new reactivities of elemental sulfur applied to
organic synthesis, we have noticed that some reactions with this element proceeded
very efficiently in highly concentrated media or even in the absence of solvent. When the
reactions were performed at high temperatures, the reaction media were in general liquid,
and the stirring was possible without any difficulty. However, if the reactions were
carried out with solid starting materials (including solid sulfur), the addition of an inert
or weakly interacting solvent could be performed to facilitate the stirring. Such solvents
should be used in sufficient quantity to avoid excessive dilution, which in turn reduces the
reactivity significantly.
Experiments
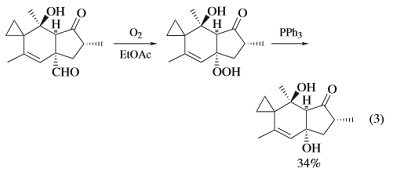
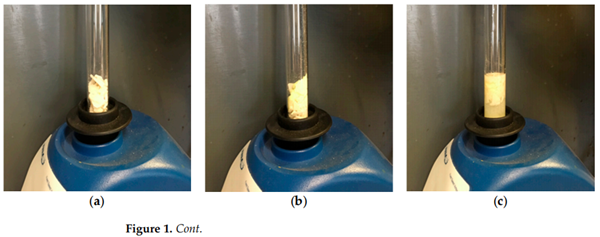
Our reaction is visualized in Figure 1. The reaction could be performed in a test
tube, although larger reactors could be used for larger scales (100 mmol). Equimolar
amounts (10 mmol) of solid triphenylphosphine (as flakes) 1a and sulfur were added to
a test tube (Figure 1a,b). The solvent (5 mL was added) (Figure 1c). We noticed that
common laboratory solvents such as CH2Cl2, CHCl3, toluene . . . were all suitable for
this reaction. The reaction tube was shaken vigorously with a vortex mixer. The reaction
mixture became rapidly homogeneously with a slight increase in temperature (about 40 ◦C).
The solid triphenylphosphine sulfide product was precipitated out after 40 s of shaking as
a white crystalline powder. The reaction mixture cooled down to rt was filtered, washed
with methanol (2 mL × 3) and dried to afford the product as a white crystalline solid
triphenylphosphine sulfide 2a (yield = 2.59 g, 88%). The filtrate could be concentrated to
provide a pure product. The purity of the product could be confirmed by 1H, 31P and 13C
NMR spectra as well as by elemental analysis. Compared to 31P NMR of the starting PPh3
(7.32 ppm in CDCl3), the chemical shift of the corresponding PPh3S is significantly different
(43.3 ppm). Compared to known methods, ours is highlighted by its high atom-efficiency
with short reaction time (<1 min) under room temperature conditions with a very small
amount of moderately polar solvents such as chloroform, CH2Cl2, toluene and its easy
purification by simple filtration.

Results and Discussion
Mechanistically, the reaction could be initiated by a nucleophilic attack of triphenylphosphine to S8 molecules to provide zwitterion A (Scheme 1), as previously proposed
by Bartlett and Meguerian. A cascade of seven-time attacks of the other seven molecules
of Ph3P would consume all the polysulfide chain. The first step could be rate determining
and depends on the concentration of both starting materials. This suggests that the reaction
is best performed under highly concentrated conditions. Since sulfur is not well soluble
in common protic polar solvents such as alcohol (methanol or ethanol) or dipolar aprotic
solvents (DMF, DMSO), the reaction performed in these solvents did not succeed in the
same manner or required longer reaction times.

The reaction conditions could also be extended to other analogs as exemplified by
two derivatives of triphenylphosphines bearing a para substituent such as chloro (1b) or
methoxy (1c). In both cases tested, the reactions on a smaller scale (1 mmol) could be conveniently performed in a 7-mL test tube with magnetic stirring and provided the
corresponding products 2b-2c in quantitative yields (See Supplementary Material).
Conclusions
The salient aspects of our developed synthetic procedure are its high atom-efficiency,
its short reaction time (<1 min), its room temperature conditions, the involvement of only a
very small amount of moderately polar solvents such as halogenated solvents (chloroform,
CH2Cl2) or aromatic hydrocarbon solvents (toluene, xylenes) and its easy purification by
simple filtration.
As a matter of fact, the purification step, especially in large-scale production, may be time-consuming, difficult or even impossible using other chromatography techniques than simple washing with solvents. This purification technique is once again demonstrated to be easily achieved. Previously, some organic reactions with elemental sulfur leading to complex scaffolds could be purified by applying the same technique.
References:
[1] NGUYEN T. Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine[J]. Clean Technologies and Environmental Policy, 2022. DOI:10.3390/cleantechnol4020013.
Related articles And Qustion
See also
Lastest Price from Triphenylphosphine manufacturers
US $0.00/KG2025-05-21
- CAS:
- 603-35-0
- Min. Order:
- 1000KG
- Purity:
- 99.5
- Supply Ability:
- 20000MT

US $0.00/kg2025-05-21
- CAS:
- 603-35-0
- Min. Order:
- 1000kg
- Purity:
- 99.5
- Supply Ability:
- 20000mt