What can 4-iodobenzaldehyde be used for?
4-Iodobenzaldehyde is an important organic building block, which has been used in the preparation of benzaldimine monolayers, 4-[2-(trimethylsilyl)ethynyl] benzaldehyde, and other substituted benzene products.
Applications
The following example is about its application on the synthesis of antibacterial agents [1]
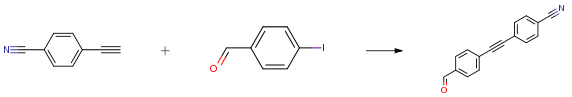
To a solution of 4-ethynylbenzonitrile (2.27g, 17.9 mmol), 4- iodobenzaldehyde (5.40 g, 23.3) and triethylamine (7.0 ml, 2.3 mmol) in THF (anh., 360 ml) was added mixture of PdCl2(PPh3)2 (400 mg, 0.6 mmol) and CuI (216 mg, 1.1 mmol). Reaction mixture was stirred at ambient temperature overnight. Solvents were evaporated in vacuum. Residue was dissolved in EtOAc (150 ml) and extracted with 5percent aq. NaHCO3 (50 ml x 2) and brine (50 ml). The organic layer was dried over anh. Na2SO4 and evaporated in vacuum. Residue was dried in vacuum overnight at ambient temperature to provide the target material (3.75 g, 90percent) as yellow solid.
The following example is about its application on the synthesis of polycyclic estrogen receptor modulators [2]
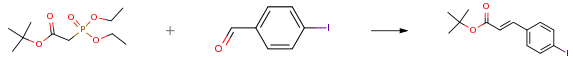
A round-bottom flask equipped with a magnetic stir bar, an addition funnel, a rubber septum, and N2 inlet was charged with 4-iodobenzaldehyde (10.0 g, 43.1 mmol), tert-butyl diethylphosphonoacetate (12.1 mL, 51.7 mmol), lithium chloride (3.7 g, 86.2 mmol) and anhydrous acetonitrile (86 mL). To this mixture, DBU (7.1 mL, 47.4 mmol) in anhydrous acetonitrile (14 mL) was slowly added drop wise via addition funnel and stirred at room temperature for 1.5 h. Upon completion, the reaction was concentrated and redissolved in dichloromethane and water. The layers were separated and the organic layer was washed with brine, dried over sodium sulfate and filtered. The crude material was concentrated and purified on a silica gel column eluted with 0-5 percent ethyl acetate in hexanes affording the product as pale yellow foam (13.2 g, 93 percent).
The following example is about its application on the synthesis of anti-estrogenic compounds [3]
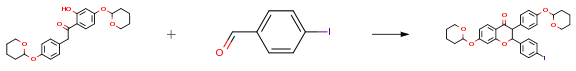
1-(2-Hydroxy-4-((tetrahydro-2H-pyran-2-yl)oxy)phenyl)-2-(4-((tetrahydro-2H-pyran-2-yl)oxy)phenyl)ethanone (293.0 g, 0.71 mol, 1.0 equiv.) was added to a three-neck 5 L round bottom flask. 2-Butanol (1.25 L) and 97.0% 4-iodobenzaldehyde (169.9 g, 0.71 mol, 1.0 equiv.) were added to the flask to provide a suspension. Piperidine (23.5 mL, 0.24 mol, 0.3 equiv.) and DBU (36.4 mL, 0.24 mol, 0.3 equiv.) were added to the suspension. The flask was fitted with a Dean-Stark apparatus, a condenser, a thermometer with an inlet adapter, and a stir bar. The reaction was heated under a nitrogen atmosphere with a mantle to provide an orange solution at 78° C. Heating was continued to reflux. Half the solvent (610 mL) was collected over 1.5 h. The reaction was heated for another hour without collecting 2-butanol. The solution gradually darkened to a red color. The mantle was removed and the flask was allowed to cool to 90° C. 2-propanol (500 mL) was then added and the reaction mixture stirred. A large mass formed at the bottom of the flask when the temperature dropped below 50° C.
The reaction was stirred for 2 days. The flask contained a solid cake and a clear solution. The supernatant was decanted and the solid was mixed with 2-propanol (100 mL) and diethyl ether (80 mL) and slowly warmed with agitation using a metal spatula until the mixture stirred freely with a stir bar. The solid mass became a viscous suspension. 25% diethyl ether/Hex (300 mL) was added to the mixture and the suspension stirred for 1 h. The suspension was filtered and the solid washed with 25% diethyl ether/Hex to provide the title compound as a white powder (240.0 g, 54%). The filtrate was concentrated and precipitation was performed, as above, to provide further compound (40.0 g, 9%). The filtrates and the supernatant were combined, concentrated, adsorbed onto silica gel and purified on silica gel with 30% EA/Hex to provide the product as a yellow foam (137.0 g, 31%). Overall yield 94%.
References
1.Achaogen, Inc. Antibacterial Agents. WO2008/154642[P], 2008, A2, Page column 196-197.
2.Seragon Pharmaceuticals, Inc. Smith ND, Govek SP, Kahraman M, Bonnefous C, Julien JD. Polycyclic estrogen receptor modulators and uses thereof. WO2014/151899[P], 2014, A1, Paragraph 00388
3.Pfizer Inc. Kushner PJ, Myles DC, Harmon CL, Hodges G, Leslie C. Anti-Estrogenic Compounds. US2016/311805[P], 2016, A1, Paragraph 0218-0221.
4. https://pubchem.ncbi.nlm.nih.gov/compound/96657
5. http://www.chemspider.com/Chemical-Structure.87265.html
);You may like
See also
Lastest Price from 4-IODOBENZALDEHYDE manufacturers

US $0.00-0.00/KG2024-02-01
- CAS:
- 15164-44-0
- Min. Order:
- 10mg
- Purity:
- 99%HPLC
- Supply Ability:
- 2000tons
US $0.00/KG2022-02-09
- CAS:
- 15164-44-0
- Min. Order:
- 1KG
- Purity:
- 97.3%
- Supply Ability:
- 100 tons

![50-32-8 Benzo[a]pyrene; PAHs; carcinogen; exposure](httpss://www.chemicalbook.com/NewsImg/2019-12-25/20191225918146735.jpg)